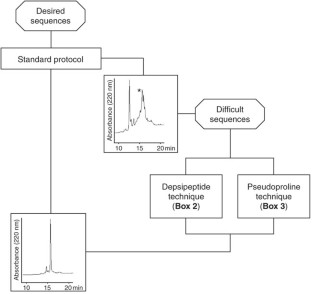
Abstract
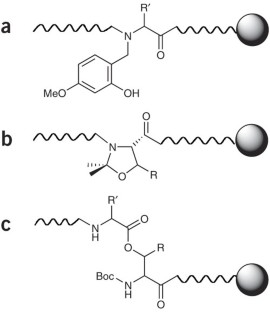
This protocol for solid-phase peptide synthesis (SPPS) is based on the widely used Fmoc/tBu strategy, activation of the carboxyl groups by aminium-derived coupling reagents and use of PEG-modified polystyrene resins. A standard protocol is described, which was successfully applied in our lab for the synthesis of the corticotropin-releasing factor (CRF), >400 CRF analogs and a countless number of other peptides. The 41-mer peptide CRF is obtained within ∼80 working hours. To achieve the so-called difficult sequences, special techniques have to be applied in order to reduce aggregation of the growing peptide chain, which is the main cause of failure for peptide chemosynthesis. Exemplary application of depsipeptide and pseudoproline units is shown for synthesizing an extremely difficult sequence, the Asn(15) analog of the WW domain FBP28, which is impossible to obtain using the standard protocol.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Fischer, E. & Otto, E. Synthesis of the derivatives of some dipeptides. Ber. Deutsch. Chem. Ges. 36, 2106–2116 (1903).
Bergmann, M. & Zervas, L. A general procedure of the peptide synthesis. Ber. Deutsch. Chem. Ges. 65, 1192–1201 (1932).
Anderson, G.W., Blodinger, J. & Welcher, A.D. Tetraethylpyrophosphite as a reagent for peptide syntheses. J. Am. Chem. Soc. 74, 5309–5311 (1952).
Siffert, R.H. & du Vigneaud, V. A new synthesis of carnosine, with some observations on the splitting of the benzyl group from carbobenzoxy derivatives and from benzylthioethers. J. Biol. Chem. 108, 753–761 (1935).
Merrifield, R.B. History of protein synthesis. In Houben-Weyl. Methods of Organic Chemistry. Vol. E 22b: Synthesis of Peptides and Peptidomimetics (eds. Goodman, M., Felix, A., Moroder, L. & Toniolo, C.) 3–41 (Thieme, Stuttgart, New York, 2002).
Du Vigneaud, V., Ressler, C., Swan, J.M., Roberts, C.W. & Katsoyannis, P.G. The synthesis of oxytocin. J. Am. Chem. Soc. 76, 3115–3121 (1954).
Merrifield, R.B. Solid phase peptide synthesis. 1. Synthesis of a tetrapeptide. J. Am. Chem. Soc. 85, 2149–2154 (1963).
Atherton, E., Clive, D.L. & Sheppard, R.C. Polyamide supports for polypeptide-synthesis. J. Am. Chem. Soc. 97, 6584–6585 (1975).
Atherton, E., Brown, E. & Sheppard, R.J. Internal association in solid-phase peptide-synthesis—synthesis of cytochrome-C residues 66-104 on polyamide supports. J. Chem. Soc. Chem. Commun. 1151–1152 (1981).
Bayer, E. Towards the chemical synthesis of proteins. Angew. Chem. Int. Ed. Engl. 30, 113–129 (1991).
Zalipsky, S., Chang, J.L., Albericio, F. & Barany, G. Preparation and applications of polyethylene glycol-polystyrene graft resin supports for solid-phase peptide-synthesis. React. Polym. 22, 243–258 (1994).
Albericio,, F. & Giralt, E. Handles and supports. In Houben-Weyl. Methods of Organic Chemistry. Vol. E 22a: Synthesis of Peptides and Peptidomimetics (eds. Goodman, M., Felix, A., Moroder, L. & Toniolo, C.) 685–709 (Thieme, Stuttgart, New York, 2002).
Carpino, L.A. Oxidative reactions of hydrazines. 2. Isophthalimides. New protective groups on nitrogen. J. Am. Chem. Soc. 79, 98–101 (1957).
Stewart, J.M. Protection strategies. In Houben-Weyl. Methods of Organic Chemistry. Vol. E 22a: Synthesis of Peptides and Peptidomimetics (eds. Goodman, M., Felix, A., Moroder, L. & Toniolo, C.) 726–739 (Thieme, Stuttgart, New York, 2002.
Carpino, L.A. 9-Fluorenylmethoxycarbonyl function, a new base-sensitive amino-protecting group. J. Am. Chem. Soc. 92, 5748 (1970).
Chang, C.D. & Meienhofer, J. Solid-phase peptide-synthesis using mild base cleavage of N-alphafluorenylmethyloxycarbonylamino acids, exemplified by a synthesis of dihydrosomatostatin. Int. J. Pept. Protein Res. 11, 246–249 (1978).
Atherton, E. & Wellings, D.A. 9-Fluorenylmethoxycarbonyl/tert-butyl strategy. In Houben-Weyl. Methods of Organic Chemistry. Vol. E 22a: Synthesis of Peptides and Peptidomimetics (eds. Goodman, M., Felix, A., Moroder, L. & Toniolo, C.) 740–754 (Thieme, Stuttgart, New York, 2002).
Sheehan, J.C. & Hess, G.P. A new method of forming peptide bonds. J. Am. Chem. Soc. 77, 1067–1068 (1955).
Wieland, T., Kern, W. & Sehring, R. Über anhydride von acylierten aminosäuren. Justus Liebigs Ann. Chem. 569, 117–121 (1950).
Schwyzer, R., Iselin, B. & Feurer, M. Über aktivierte ester. 1. Aktivierte ester der hippursäure und ihre umsetzungen mit benzylamin. Helv. Chim. Acta 38, 69–79 (1955).
Coste, J. Phosphonium salts. In Houben-Weyl. Methods of Organic Chemistry. Vol. E 22a: Synthesis of Peptides and Peptidomimetics (eds. Goodman, M., Felix, A., Moroder, L. & Toniolo, C.) 538–554 (Thieme, Stuttgart, New York, 2002).
Bienert, M., Henklein, P., Beyermann, M. & Carpino, L. A. Uronium/guanidinium salts. In Houben-Weyl. Methods of Organic Chemistry. Vol. E 22a: Synthesis of Peptides and Peptidomimetics (eds. Goodman, M., Felix, A., Moroder, L. & Toniolo, C.) 555–580 (Thieme, Stuttgart, New York, 2002).
Atherton, E. & Sheppard, R.C. Solid Phase Peptide Synthesis: A Practical Approach (IRL Press, Oxford, UK, 1999).
Pennington, M.W. & Dunn, B.M. Peptide Synthesis Protocols (Humana Press, Totowa, New Jersey, 1994).
Fields, G.B. Solid-Phase Peptide Synthesis (Academic Press, New York, 1997).
Lloyd-Williams, P., Albericio, F. & Giralt, E. Chemical Approaches to the Synthesis of Peptides and Proteins (CRC Press, Boca Raton, Florida, 1997).
Chan, W.C. & White, P.D. Fmoc Solid Phase Peptide Synthesis: A Practical Approach (Oxford University Press, Oxford, UK, 2000).
Sewald, N. & Jakubke,, H.-D. Peptides: Chemistry and Biology (Wiley-VCH, Weinheim, 2002).
Goodman, M., Felix, A., Moroder, L. & Toniolo, C. (eds.) Houben-Weyl. Methods of Organic Chemistry. Vol. E 22a–e: Synthesis of Peptides and Peptidomimetics (Thieme, Stuttgart, New York, 2002).
Amblard, M., Fehrentz, J.A., Martinez, J. & Subra, G. Methods and protocols of modern solid phase peptide synthesis. Mol. Biotechnol. 33, 239–254 (2006).
Dawson, P.E. & Kent, S.B. Synthesis of native proteins by chemical ligation. Annu. Rev. Biochem. 69, 923–960 (2000).
Bray, B.L. Large-scale manufacture of peptide therapeutics by chemical synthesis. Nat. Rev. Drug Discovery 2, 587–593 (2003).
Vale, W., Spiess, J., Rivier, C. & Rivier, J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science 213, 1394–1397 (1981).
Dauzenberg, F.M. & Hauger, R.L. The CRF peptide family and their receptors: yet more partners discovered. Trends Pharmacol. Sci. 23, 71–77 (2002).
Beyermann, M., Fechner, K., Furkert, J., Krause, E. & Bienert, M. A single-point slight alteration set as a tool for structure-activity relationship studies of ovine corticotropin- releasing factor. J. Med. Chem. 39, 3324–3330 (1996).
Beyermann, M. et al. A role for a helical connector between two receptor binding sites of a long-chain peptide hormone. J. Biol. Chem. 275, 5702–5709 (2000).
Beyermann, M. et al. Achieving signalling selectivity of ligands for the corticotropin-releasing factor type 1 receptor by modifying the agonist's signalling domain. Br. J. Pharmacol. 151, 851–859 (2007).
Rivier, J.E. & Miranda, M.T.M. Solid-phase peptide synthesis at elevated temperature. In Houben-Weyl. Methods of Organic Chemistry. Vol. E 22a: Synthesis of Peptides and Peptidomimetics (eds. Goodman, M., Felix, A., Moroder, L. & Toniolo, C.) 806–813 (Thieme, Stuttgart, New York, 2002).
Kaiser, E., Colescot, R.L., Bossinge, C.D. & Cook, P.I. Color test for detection of free terminal amino groups in solid-phase synthesis of peptides. Anal. Biochem. 34, 595–598 (1970).
Carpino, L.A., Beyermann, M., Wenschuh, H. & Bienert, M. Peptide synthesis via amino acid halides. Acc. Chem. Res. 29, 268–274 (1996).
Gilon, C., Dechantsreiter, M.A., Burkhart, F., Friedler, A. & Kessler, H. Synthesis of N-alkylated peptides. In Houben-Weyl. Methods of Organic Chemistry. Vol. E 22c: Synthesis of Peptides and Peptidomimetics (eds. Goodman, M., Felix, A., Moroder, L. & Toniolo, C.) 215–271 (Thieme, Stuttgart, New York, 2002).
Hyde, C., Johnson, T., Owen, D., Quibell, M. & Sheppard, R.C. Some “difficult sequences” made easy. A study of interchain association in solid-phase peptide synthesis. Int. J. Peptide Protein Res. 43, 431–440 (1994).
Fields, C. & Fields, G.B. Solvents for solid-phase peptide synthesis. In Peptide Synthesis Protocols (eds. Penningten, M.W. & Dunn, B.M.) 29–40 (Humana Press, Totowa, New Jersey, 1994).
Beyermann, M. & Bienert, M. Synthesis of difficult peptide sequences: a comparison of Fmoc- and Boc-technique. Tetrahedron Lett. 33, 3745–3748 (1992).
Narita, M., Fukunaga, T., Wakabayashi, A., Ishikawa, K. & Nakano, H. Syntheses and properties of tertiary peptide bond-containing polypeptides. 1. Syntheses and properties of oligo(L-leucine)S containing proline of glycyl-N-(2,4-dimethoxybenzyl)-L-leucine residues. Int. J. Peptide Protein Res. 23, 306–314 (1984).
Johnson, T., Quibell, M. & Sheppard, R.C. N,O-bis Fmoc derivatives of N-(2-hydroxy-4-methoxybenzyl)-amino acids: useful intermediates in peptide synthesis. J. Pept. Sci. 1, 11–25 (1995).
Quibell, M., Turnell, W.G. & Johnson, T. Preparation and purification of beta-amyloid (1-43) via soluble, amide backbone protected intermediates. J. Org. Chem. 59, 1745–1750 (1994).
Wöhr, T. & Mutter, M. Pseudo-prolines in peptide synthesis: direct insertion of serine and threonine-derived oxazolidines in dipeptides. Tetrahedron Lett. 36, 3847–3848 (1995).
Wöhr, T. et al. Pseudo-prolines as a solubilizing, structure-disrupting protection technique in peptide synthesis. J. Am. Chem. Soc. 118, 9218–9227 (1996).
Toniolo, C., Bonora, G.M., Mutter, M. & Pillai, V.N.R. Linear oligopeptides. 78. The effect of the insertion of a proline residue on the solution conformation of host peptides. Macromol. Chem. Phys. 182, 2007–2014 (1981).
Carpino, L.A. et al. Synthesis of “difficult” peptide sequences: application of a depsipeptide technique to the Jung-Redemann 10- and 26-mers and the amyloid peptide Aβ(1-42). Tetrahedron Lett. 45, 7519–7523 (2004).
Mutter, M. et al. Switch peptides in statu nascendi: induction of conformational transitions relevant to degenerative diseases. Angew. Chem. Int. Ed. Engl. 43, 4172–4178 (2004).
Sohma, Y., Sasaki, M., Hayashi, Y., Kimura, T. & Kiso, Y. Design and synthesis of a novel water-soluble Aβ(1-42) isopeptide: an efficient strategy for the preparation of Alzheimer's disease-related peptide, Aβ(1-42), via O-N intramolecular acyl migration reaction. Tetrahedron Lett. 45, 5965–5968 (2004).
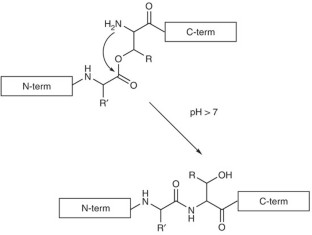
Coin, I. et al. Depsipeptide methodology for solid-phase peptide synthesis: circumventing side reactions and development of an automated technique via depsidipeptide units. J. Org. Chem. 71, 6171–6177 (2006).
Sohma, Y. et al. 'O-Acyl isopeptide method' for the efficient synthesis of difficult sequence-containing peptides: use of 'O-acyl isodipeptide unit'. Tetrahedron Lett. 47, 3013–3017 (2006).
Coin, I., Schmieder, P., Bienert, M. & Beyermann, M. The depsipeptide technique applied to peptide segment condensation: scope and limitations. J. Pept. Sci. DOI: 10.1002/psc.928 (2007).
Taniguchi, A. et al. 'O-Acyl isopeptide method' for peptide synthesis: solvent effects in the synthesis of Aβ1-42 isopeptide using 'O-acyl isodipeptide unit'. J. Pept. Sci. DOI: 10.1002/psc.905 (2007).
Pedroso, E., Grandas, A., de las Heras, X., Eritja, R. & Girald, E. Diketopiperazine formation in solid-phase peptide-synthesis using P-alkoxybenzyl ester resins and Fmoc-amino acids. Tetrahedron Lett. 27, 743–746 (1986).
Carpino, L.A. et al. New family of base- and nucleophile-sensitive amino-protecting groups. A Michael-acceptor-based deblocking process. Practical utilization of the 1,1-dioxobenzo[b]thiophene-2-ylmethyloxycarbonyl (Bsmoc)group. J. Am. Chem. Soc. 119, 9915–9916 (1997).
Fujino, M., Wakimasu, M., Shinagawa, S., Kitada, C. & Yajima, H. Synthesis of the nonacosapeptide corresponding to mammalian glucagons. Chem. Pharm. Bull. 26, 539–548 (1978).
Marcias, M.J., Gervais, V., Civera, C. & Oschkinat, H. Structural analysis of WW domains and design of a WW prototype. Nat. Struct. Biol. 7, 375–379 (2000).
Tremmel, S. et al. C-13-labeled tyrosine residues as local IR probes for monitoring conformational changes in peptides and proteins. Angew. Chem. Int. Ed. Engl. 44, 4631–4635 (2005).
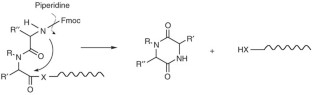
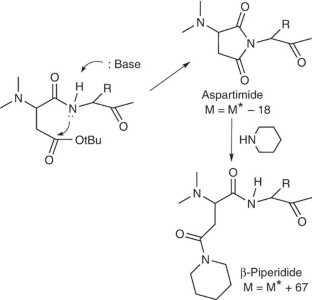
Nicolás, E., Pedroso, E. & Girald, E. Formation of aspartimide peptides in Asp-Gly sequences. Tetrahedron Lett. 30, 497–500 (1989).
Dölling, R. et al. Piperidine-mediated side product formation for Asp(OtBu)-containing peptides. J. Chem. Soc. Chem. Commun. 853–854 (1994).
Offer, J., Quibell, M. & Johnson, T. On-resin solid-phase synthesis of asparagine N-linked glycopeptides: use of N-(2-acetoxy-4-methoxybenzyl)(AcHmb) aspartyl amide-bond protection to prevent unwanted aspartimide formation. J. Chem. Soc. Perkin Trans. 1, 175–182 (1996).
Wade, J.D., Bedford, J., Sheppard, R.C. & Tregear, G.W. DBU as an N-alpha-deprotecting reagent for the fluorenylmethoxycarbonyl group in continuous flow solid-phase peptide synthesis. Pept. Res. 4, 194–199 (1991).
Kates, S.A., Solé, N.A., Beyermann, M., Barany, G. & Albericio, F. Optimized preparation of deca(L-alanyl)-L-valinamide by 9-fluorenylmethyloxycarbonyl (Fmoc) solid-phase synthesis on polyethylene glycol-polystyrene (PEG-PS) graft supports, with 1,8-diazobicyclo[5.4.0]-undec-7-ene (DBU) deprotection. Pept. Res. 9, 106–113 (1996).
Thaler, A., Seebach, D. & Cardinaux, F. Improvement of degree of resin swelling and of efficiency of coupling in solid-phase synthesis. Helv. Chim. Acta 74, 628–643 (1991).
Pugh, K.C., York, E.J. & Stewart, J.M. Effects of resin swelling and substitution on solid phase synthesis. Int. J. Pept. Protein Res. 40, 208–213 (1992).
Pennington, M.W. & Byrnes, M.E. Procedures to improve difficult couplings. In Peptide Synthesis Protocols (eds. Pennington, M.W. & Dunn, B.M.) 1–16 (Humana Press, Totowa, New Jersey, 1994).
Wade, J.D., Mathieu, M.N., Macris, M. & Tregear, G.W. Base-induced side reactions in Fmoc-solid phase peptide synthesis: minimization by use of piperazine as N-alpha-deprotection reagent. Lett. Pept. Sci. 7, 107–112 (2000).
Alsina, J., Giralt, E. & Albericio, F. Use of N-tritylamino acids and PyAOP for the supression of diketopiperazine formation in Fmoc/(t)Bu solid-phase peptide synthesis using alkoxybenzyl ester anchoring linkages. Tetrahedron Lett. 37, 4195–4198 (1996).
Schnölzer, M., Alewood, P., Jones, A., Alewood, D. & Kent, S.B.H. In situ neutralization in Boc-chemistry solid-phase peptide synthesis—rapid, high-yield assembly of difficult sequences. Int. J. Peptide Protein Res. 40, 180–193 (1992).
Krause, E. et al. Studies on thioether modifications: S-oxidation, S-oxide reduction and regeneration of methionine peptides from their S-benzyl-sulfonium derivatives. In Peptides: Chemistry and Biology (eds. Smith, J.A. & Rivier, J.E.) 478–479 (ESCOM, Leiden, the Netherlands, 1992).
Acknowledgements
We gratefully acknowledge contributions of S. Tremmel, E. Krause, C.D. Sferdean and L.A. Carpino. We thank A. Klose and D. Krause for technical assistance. This work was supported by the Deutsche Forschungsgemeinschaft, grant no. FOR 299/2-2 TP2 and Be 1434/5-2.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Coin, I., Beyermann, M. & Bienert, M. Solid-phase peptide synthesis: from standard procedures to the synthesis of difficult sequences. Nat Protoc 2, 3247–3256 (2007). https://doi.org/10.1038/nprot.2007.454
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2007.454
This article is cited by
-
Influence of the solvent removal method on the morphology of polystyrene porous structures prepared via thermally induced phase separation
Journal of Porous Materials (2024)
-
Antimicrobial peptide moricin induces ROS mediated caspase-dependent apoptosis in human triple-negative breast cancer via suppression of notch pathway
Cancer Cell International (2023)
-
Novel, Robust and Efficient W/Co@g-C3N4 Catalyst Enable Outstanding Performance for the Straightforward Oxidative Amidation of Aldehydes with Amines
Catalysis Letters (2023)
-
Solution NMR Studies of LPRDA Peptide: an Oligopeptide Inhibitor of Staphylococcus aureus Sortase A
Applied Magnetic Resonance (2023)
-
Low-Loaded Polyethylene Glycol (PEG) Resin for High-Purity Peptide Synthesis and Cell Binding Assays
BioChip Journal (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.



