Abstract
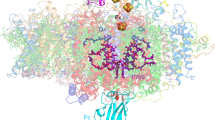
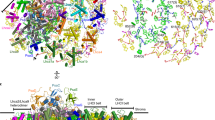
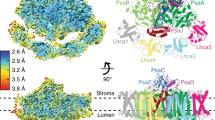
Four elaborate membrane complexes carry out the light reaction of oxygenic photosynthesis. Photosystem I (PSI) is one of two large reaction centres responsible for converting light photons into the chemical energy needed to sustain life. In the thylakoid membranes of plants, PSI is found together with its integral light-harvesting antenna, light-harvesting complex I (LHCI), in a membrane supercomplex containing hundreds of light-harvesting pigments. Here, we report the crystal structure of plant PSI–LHCI at 2.6 Å resolution. The structure reveals the configuration of PsaK, a core subunit important for state transitions in plants, a conserved network of water molecules surrounding the electron transfer centres and an elaborate structure of lipids bridging PSI and its LHCI antenna. We discuss the implications of the structure for energy transfer and the evolution of PSI.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Blankenship, R. E. Origin and early evolution of photosynthesis. Photosynth. Res. 33, 91–111 (1992).
Barber, J. Engine of life and big bang of evolution: a personal perspective. Photosynth. Res. 80, 137–155 (2004).
Nelson, N. Evolution of photosystem I and the control of global enthalpy in an oxidizing world. Photosynth. Res. 116, 145–151 (2013).
Rensing, S. A. et al. The Physcomitrella genome reveals evolutionary insights into the conquest of land by plants. Science 319, 64–69 (2008).
Nelson, N. & Yocum, C. F. Structure and function of photosystems I and II. Annu. Rev. Plant Biol. 57, 521–565 (2006).
Croce, R. & van Amerongen, H. Light-harvesting in photosystem I. Photosynth. Res. 116, 153–166 (2013).
Nelson, N. & Junge, W. Structure and energy transfer in photosystems of oxygenic photosynthesis. Annu. Rev. Biochem. 84, 659–683 (2015).
Jordan, P. et al. Three-dimensional structure of cyanobacterial photosystem I at 2.5 Å resolution. Nature 411, 909–917 (2001).
Ben-Shem, A., Frolow, F. & Nelson, N. Crystal structure of plant photosystem I. Nature 426, 630–635 (2003).
Umena, Y., Kawakami, K., Shen, J. R. & Kamiya, N. Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å. Nature 473, 55–60 (2011).
Wei, X. et al. Structure of spinach photosystem II-LHCII supercomplex at 3.2 Å resolution. Nature 534, 69–74 (2016).
Mazor, Y., Borovikova, A. & Nelson, N. The structure of plant photosystem I super-complex at 2.8 Å resolution. eLife 4, e07433 (2015).
Qin, X., Suga, M., Kuang, T. & Shen, J. R. Photosynthesis. Structural basis for energy transfer pathways in the plant PSI–LHCI supercomplex. Science 348, 989–995 (2015).
Le Quiniou, C. et al. PSI–LHCI of Chlamydomonas reinhardtii: increasing the absorption cross section without losing efficiency. Biochim. Biophys. Acta 1847, 458–467 (2015).
Rochaix, J. D. Regulation and dynamics of the light-harvesting system. Annu. Rev. Plant Biol. 65, 287–309 (2014).
Weinert, T. et al. Fast native-SAD phasing for routine macromolecular structure determination. Nat. Methods 12, 131–133 (2015).
Mazor, Y., Nataf, D., Toporik, H. & Nelson, N. Crystal structures of virus-like photosystem I complexes from the mesophilic cyanobacterium Synechocystis PCC 6803. eLife 3, e01496 (2014).
Guergova-Kuras, M., Boudreaux, B., Joliot, A., Joliot, P. & Redding, K. Evidence for two active branches for electron transfer in photosystem I. Proc. Natl Acad. Sci. USA 98, 4437–4442 (2001).
Sharon, I. et al. Photosystem I gene cassettes are present in marine virus genomes. Nature 461, 258–262 (2009).
Jensen, P. E., Gilpin, M., Knoetzel, J. & Scheller, H. V. The PSI-K subunit of photosystem I is involved in the interaction between light-harvesting complex I and the photosystem I reaction center core. J. Biol. Chem. 275, 24701–24708 (2000).
Kouril, R. et al. Structural characterization of a complex of photosystem I and light-harvesting complex II of Arabidopsis thaliana. Biochemistry 44, 10935–10940 (2005).
Gounaris, K. & Barber, J. Monogalactosyldiacylglycerol: the most abundant polar lipid in nature. Trends Biochem. Sci. 8, 378–381 (1983).
Amunts, A., Drory, O. & Nelson, N. The structure of a plant photosystem I supercomplex at 3.4 Å resolution. Nature 447, 58–63 (2007).
Sato, N., Suda, K. & Tsuzuki, M. Responsibility of phosphatidylglycerol for biogenesis of the PSI complex. Biochim. Biophys. Acta 1658, 235–243 (2004).
Block, M. A., Douce, R., Joyard, J. & Rolland, N. Chloroplast envelope membranes: a dynamic interface between plastids and the cytosol. Photosynth. Res. 92, 225–244 (2007).
Bishop, D. G., Kenrick, J. R., Bayston, J. H., Macpherson, A. S. & Johns, S. R. Monolayer properties of chloroplast lipids. Biochim. Biophys. Acta 602, 248–259 (1980).
Jansson, S. A guide to the Lhc genes and their relatives in Arabidopsis. Trends Plant. Sci. 4, 236–240 (1999).
Kargul, J. et al. Light-harvesting complex II protein CP29 binds to photosystem I of Chlamydomonas reinhardtii under State 2 conditions. FEBS J. 272, 4797–4806 (2005).
Liu, Z. et al. Crystal structure of spinach major light-harvesting complex at 2.72 Å resolution. Nature 428, 287–292 (2004).
Standfuss, J., Terwisscha van Scheltinga, A. C., Lamborghini, M. & Kühlbrandt, W. Mechanisms of photoprotection and nonphotochemical quenching in pea light-harvesting complex at 2.5 Å resolution. EMBO J. 24, 919–928 (2005).
Wientjes, E., van Stokkum, I. H., van Amerongen, H. & Croce, R. Excitation-energy transfer dynamics of higher plant photosystem I light-harvesting complexes. Biophys. J. 100, 1372–1380 (2011).
Morosinotto, T., Breton, J., Bassi, R. & Croce, R. The nature of a chlorophyll ligand in Lhca proteins determines the far red fluorescence emission typical of photosystem I. J. Biol. Chem. 278, 49223–9 (2003).
Morosinotto, T., Mozzo, M., Bassi, R. & Croce, R. Pigment-pigment interactions in Lhca4 antenna complex of higher plants photosystem I. J. Biol. Chem. 280, 20612–20619 (2005).
Mozzo, M., Morosinotto, T., Bassi, R. & Croce, R. Probing the structure of Lhca3 by mutation analysis. Biochim. Biophys. Acta. 1757, 1607–1613 (2006).
Wientjes, E., Roest, G. & Croce, R. From red to blue to far-red in Lhca4: how does the protein modulate the spectral properties of the pigments? Biochim. Biophys. Acta. 1817, 711–717 (2012).
Morosinotto, T., Castelletti, S., Breton, J., Bassi, R. & Croce, R. Mutation analysis of Lhca1 antenna complex. Low energy absorption forms originate from pigment-pigment interactions. J. Biol. Chem. 277, 36253–36261 (2002).
Förster, T. Ein beitrag zur theorie der photosynthese. Z. Naturforsch. 2b, 174–182 (1947).
Gradinaru, C. C. et al. The flow of excitation energy in LHCII monomers: implications for the structural model of the major plant antenna. Biophys. J. 75, 3064–3077 (1998).
van Oort, B. et al. Picosecond fluorescence of intact and dissolved PSI–LHCI crystals. Biophys. J. 95, 5851–5861 (2008).
Foadi, J. et al. Clustering procedures for the optimal selection of data sets from multiple crystals in macromolecular crystallography. Acta Crystallogr. D 69, 1617–1632 (2013).
Giordano, R., Leal, R. M., Bourenkov, G. P., McSweeney, S. & Popov, A. N. The application of hierarchical cluster analysis to the selection of isomorphous crystals. Acta Crystallogr. D 68, 649–658 (2012).
Pape, T. & Schneider, T. R. HKL2MAP: a graphical user interface for macromolecular phasing with SHELX programs. J. Appl. Crystallogr. 37, 843–844 (2004).
Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. A 64, 112–122 (2008).
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D 66, 213–221 (2010).
Afonine, P. V., Headd, J. J., Terwilliger, T. C. & Adams, P. D. New tool: phenix.real_space_refine. Comput. Crystallogr. News 4, 36–42 (2013).
Acknowledgements
The authors would like to thank the ESRF, SLS and BESSYII synchrotrons for beam time and the staff scientists for excellent guide and relentless help. We would like to thank O. Rog for critical reading of the manuscript. This work is supported by a grant no. 293579—HOPSEP from the European Research Council, The Israel Science Foundation through grant no. 71/14 and by the I-CORE Program of the Planning and Budgeting Committee and The Israel Science Foundation (grant no. 1775/12).
Author information
Authors and Affiliations
Contributions
Y.M and N.N. performed experiments, analysed the data and wrote the paper. I.C. analysed the data. A.B. performed experiments.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures 1–5, Supplementary Tables 1–3, Supplementary References. (PDF 1745 kb)
Rights and permissions
About this article
Cite this article
Mazor, Y., Borovikova, A., Caspy, I. et al. Structure of the plant photosystem I supercomplex at 2.6 Å resolution. Nature Plants 3, 17014 (2017). https://doi.org/10.1038/nplants.2017.14
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/nplants.2017.14
This article is cited by
-
Architecture of symbiotic dinoflagellate photosystem I–light-harvesting supercomplex in Symbiodinium
Nature Communications (2024)
-
Uncovering the photosystem I assembly pathway in land plants
Nature Plants (2024)
-
Energetic robustness to large scale structural fluctuations in a photosynthetic supercomplex
Nature Communications (2023)
-
Structural insights into a unique PSI–LHCI–LHCII–Lhcb9 supercomplex from moss Physcomitrium patens
Nature Plants (2023)
-
Structural insights into the assembly and energy transfer of the Lhcb9-dependent photosystem I from moss Physcomitrium patens
Nature Plants (2023)