Abstract
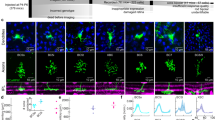
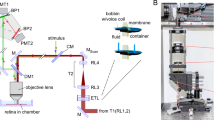
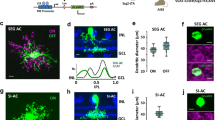
Bipolar cells form parallel channels that carry visual signals from the outer to the inner retina. Each type of bipolar cell is thought to carry a distinct visual message to select types of amacrine cells and ganglion cells. However, the number of ganglion cell types exceeds that of the bipolar cells providing their input, suggesting that bipolar cell signals diversify on transmission to ganglion cells. We explored in the salamander retina how signals from individual bipolar cells feed into multiple ganglion cells and found that each bipolar cell was able to evoke distinct responses among ganglion cells, differing in kinetics, adaptation and rectification properties. This signal divergence resulted primarily from interactions with amacrine cells that allowed each bipolar cell to send distinct signals to its target ganglion cells. Our findings indicate that individual bipolar cell–ganglion cell connections have distinct transfer functions. This expands the number of visual channels in the inner retina and enhances the computational power and feature selectivity of early visual processing.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Wässle, H. Parallel processing in the mammalian retina. Nat. Rev. Neurosci. 5, 747–757 (2004).
Masland, R.H. The fundamental plan of the retina. Nat. Neurosci. 4, 877–886 (2001).
Wu, S.M., Gao, F. & Maple, B.R. Functional architecture of synapses in the inner retina: segregation of visual signals by stratification of bipolar cell axon terminals. J. Neurosci. 20, 4462–4470 (2000).
Ghosh, K.K., Bujan, S., Haverkamp, S., Feigenspan, A. & Wässle, H. Types of bipolar cells in the mouse retina. J. Comp. Neurol. 469, 70–82 (2004).
Roska, B., Molnar, A. & Werblin, F.S. Parallel processing in retinal ganglion cells: how integration of space-time patterns of excitation and inhibition form the spiking output. J. Neurophysiol. 95, 3810–3822 (2006).
Boycott, B.B. & Wässle, H. Morphological classification of bipolar cells of the primate retina. Eur. J. Neurosci. 3, 1069–1088 (1991).
Awatramani, G.B. & Slaughter, M.M. Origin of transient and sustained responses in ganglion cells of the retina. J. Neurosci. 20, 7087–7095 (2000).
Mariani, A.P. Bipolar cells in monkey retina selective for the cones likely to be blue-sensitive. Nature 308, 184–186 (1984).
Slaughter, M.M. & Miller, R.F. 2-amino-4-phosphonobutyric acid: a new pharmacological tool for retina research. Science 211, 182–185 (1981).
DeVries, S.H. Bipolar cells use kainate and AMPA receptors to filter visual information into separate channels. Neuron 28, 847–856 (2000).
Tachibana, M. & Kaneko, A. Retinal bipolar cells receive negative feedback input from GABAergic amacrine cells. Vis. Neurosci. 1, 297–305 (1988).
Nirenberg, S. & Meister, M. The light response of retinal ganglion cells is truncated by a displaced amacrine circuit. Neuron 18, 637–650 (1997).
Dong, C.J. & Werblin, F.S. Temporal contrast enhancement via GABAC feedback at bipolar terminals in the tiger salamander retina. J. Neurophysiol. 79, 2171–2180 (1998).
Gollisch, T. & Meister, M. Eye smarter than scientists believed: neural computations in circuits of the retina. Neuron 65, 150–164 (2010).
Enroth-Cugell, C. & Freeman, A.W. The receptive-field spatial structure of cat retinal Y cells. J. Physiol. (Lond.) 384, 49–79 (1987).
Demb, J.B., Zaghloul, K., Haarsma, L. & Sterling, P. Bipolar cells contribute to nonlinear spatial summation in the brisk-transient (Y) ganglion cell in mammalian retina. J. Neurosci. 21, 7447–7454 (2001).
Baccus, S.A., Ölveczky, B.P., Manu, M. & Meister, M. A retinal circuit that computes object motion. J. Neurosci. 28, 6807–6817 (2008).
Enroth-Cugell, C. & Robson, J.G. The contrast sensitivity of retinal ganglion cells of the cat. J. Physiol. (Lond.) 187, 517–552 (1966).
Demb, J.B. Cellular mechanisms for direction selectivity in the retina. Neuron 55, 179–186 (2007).
Burrone, J. & Lagnado, L. Synaptic depression and the kinetics of exocytosis in retinal bipolar cells. J. Neurosci. 20, 568–578 (2000).
Singer, J.H. & Diamond, J.S. Vesicle depletion and synaptic depression at a mammalian ribbon synapse. J. Neurophysiol. 95, 3191–3198 (2006).
Jarsky, T. et al. A synaptic mechanism for retinal adaptation to luminance and contrast. J. Neurosci. 31, 11003–11015 (2011).
Oesch, N.W. & Diamond, J.S. Ribbon synapses compute temporal contrast and encode luminance in retinal rod bipolar cells. Nat. Neurosci. 14, 1555–1561 (2011).
Ölveczky, B.P., Baccus, S.A. & Meister, M. Retinal adaptation to object motion. Neuron 56, 689–700 (2007).
Wässle, H., Puller, C., Müller, F. & Haverkamp, S. Cone contacts, mosaics and territories of bipolar cells in the mouse retina. J. Neurosci. 29, 106–117 (2009).
Zhang, A.-J. & Wu, S.M. Receptive fields of retinal bipolar cells are mediated by heterogeneous synaptic circuitry. J. Neurosci. 29, 789–797 (2009).
Zhang, A.-J. & Wu, S.M. Responses and receptive fields of amacrine cells and ganglion cells in the salamander retina. Vision Res. 50, 614–622 (2010).
Arai, I., Tanaka, M. & Tachibana, M. Active roles of electrically coupled bipolar cell network in the adult retina. J. Neurosci. 30, 9260–9270 (2010).
Mittman, S., Taylor, W.R. & Copenhagen, D.R. Concomitant activation of two types of glutamate receptor mediates excitation of salamander retinal ganglion cells. J. Physiol. (Lond.) 428, 175–197 (1990).
Lukasiewicz, P.D., Lawrence, J.E. & Valentino, T.L. Desensitizing glutamate receptors shape excitatory synaptic inputs to tiger salamander retinal ganglion cells. J. Neurosci. 15, 6189–6199 (1995).
Jones, S.M. & Palmer, M.J. Activation of the tonic GABAC receptor current in retinal bipolar cell terminals by nonvesicular GABA release. J. Neurophysiol. 102, 691–699 (2009).
von Gersdorff, H. & Matthews, G. Depletion and replenishment of vesicle pools at a ribbontype synaptic terminal. J. Neurosci. 17, 1919–1927 (1997).
Kim, K.J. & Rieke, F. Temporal contrast adaptation in the input and output signals of salamander retinal ganglion cells. J. Neurosci. 21, 287–299 (2001).
Kim, K.J. & Rieke, F. Slow Na+ inactivation and variance adaptation in salamander retinal ganglion cells. J. Neurosci. 23, 1506–1516 (2003).
Kastner, D.B. & Baccus, S.A. Coordinated dynamic encoding in the retina using opposing forms of plasticity. Nat. Neurosci. 14, 1317–1322 (2011).
Rieke, F. Temporal contrast adaptation in salamander bipolar cells. J. Neurosci. 21, 9445–9454 (2001).
Baccus, S.A. & Meister, M. Fast and slow contrast adaptation in retinal circuitry. Neuron 36, 909–919 (2002).
Heidelberger, R., Heinemann, C., Neher, E. & Matthews, G. Calcium dependence of the rate of exocytosis in a synaptic terminal. Nature 371, 513–515 (1994).
Matsui, K., Hosoi, N. & Tachibana, M. Excitatory synaptic transmission in the inner retina: paired recordings of bipolar cells and neurons of the ganglion cell layer. J. Neurosci. 18, 4500–4510 (1998).
Geffen, M.N., de Vries, S.E.J. & Meister, M. Retinal ganglion cells can rapidly change polarity from Off to On. PLoS Biol. 5, e65 (2007).
Roska, B., Nemeth, E. & Werblin, F.S. Response to change is facilitated by a three-neuron disinhibitory pathway in the tiger salamander retina. J. Neurosci. 18, 3451–3459 (1998).
Dowling, J.E. & Werblin, F.S. Synaptic organization of the vertebrate retina. Virus Res. 11, 1–15 (1971).
Brandstätter, J.H., Koulen, P., Kuhn, R., van der Putten, H. & Wässle, H. Compartmental localization of a metabotropic glutamate receptor (mGluR7): two different active sites at a retinal synapse. J. Neurosci. 16, 4749–4756 (1996).
Taylor, W.R., Mittman, S. & Copenhagen, D.R. Passive electrical cable properties and synaptic excitation of tiger salamander retinal ganglion cells. Vis. Neurosci. 13, 979–990 (1996).
Dreosti, E., Esposti, F., Baden, T. & Lagnado, L. In vivo evidence that retinal bipolar cells generate spikes modulated by light. Nat. Neurosci. 14, 951–952 (2011).
Lagnado, L., Gomis, A. & Job, C. Continuous vesicle cycling in the synaptic terminal of retinal bipolar cells. Neuron 17, 957–967 (1996).
Cook, P.B. & McReynolds, J.S. Lateral inhibition in the inner retina is important for spatial tuning of ganglion cells. Nat. Neurosci. 1, 714–719 (1998).
Famiglietti, E.V. Polyaxonal amacrine cells of rabbit retina: morphology and stratification of PA1 cells. J. Comp. Neurol. 316, 391–405 (1992).
Barlow, H.B. & Levick, W.R. The mechanism of directionally selective units in rabbit's retina. J. Physiol. (Lond.) 178, 477–504 (1965).
Hosoya, T., Baccus, S.A. & Meister, M. Dynamic predictive coding by the retina. Nature 436, 71–77 (2005).
Meister, M., Pine, J. & Baylor, D.A. Multi-neuronal signals from the retina: acquisition and analysis. J. Neurosci. Methods 51, 95–106 (1994).
Segev, R., Goodhouse, J., Puchalla, J. & Berry, M.J. Recording spikes from a large fraction of the ganglion cells in a retinal patch. Nat. Neurosci. 7, 1154–1161 (2004).
Tsodyks, M., Pawelzik, K. & Markram, H. Neural networks with dynamic synapses. Neural Comput. 10, 821–835 (1998).
Mao, B.Q., MacLeish, P.R. & Victor, J.D. The intrinsic dynamics of retinal bipolar cells isolated from tiger salamander. Vis. Neurosci. 15, 425–438 (1998).
Kaneko, A., Pinto, L.H. & Tachibana, M. Transient calcium current of retinal bipolar cells of the mouse. J. Physiol. (Lond.) 410, 613–629 (1989).
Eggers, E.D. & Lukasiewicz, P.D. Receptor and transmitter release properties set the time course of retinal inhibition. J. Neurosci. 26, 9413–9425 (2006).
Acknowledgements
We gratefully acknowledge E. Soucy for his extensive help with the experiments, as well as all of the members of the Meister laboratory for many useful discussions. This work was supported by a Postdoctoral Fellowship for Research Abroad from the Japan Society for the Promotion of Science (H.A.) and grants from the US National Institutes of Health (M.M.).
Author information
Authors and Affiliations
Contributions
H.A. and M.M. designed the study and wrote the manuscript. H.A. performed the experiments and analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 (PDF 153 kb)
Rights and permissions
About this article
Cite this article
Asari, H., Meister, M. Divergence of visual channels in the inner retina. Nat Neurosci 15, 1581–1589 (2012). https://doi.org/10.1038/nn.3241
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3241
This article is cited by
-
A presynaptic source drives differing levels of surround suppression in two mouse retinal ganglion cell types
Nature Communications (2024)
-
Functional characterization of retinal ganglion cells using tailored nonlinear modeling
Scientific Reports (2019)
-
Assembly and maintenance of GABAergic and Glycinergic circuits in the mammalian nervous system
Neural Development (2018)
-
Inference of neuronal functional circuitry with spike-triggered non-negative matrix factorization
Nature Communications (2017)
-
Retinal bipolar cells: elementary building blocks of vision
Nature Reviews Neuroscience (2014)