Abstract
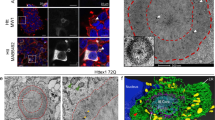
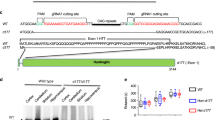
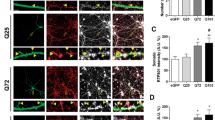
Selected vulnerability of neurons in Huntington's disease suggests that alterations occur in a cellular process that is particularly critical for neuronal function. Supporting this idea, pathogenic Htt (polyQ-Htt) inhibits fast axonal transport (FAT) in various cellular and animal models of Huntington's disease (mouse and squid), but the molecular basis of this effect remains unknown. We found that polyQ-Htt inhibited FAT through a mechanism involving activation of axonal cJun N-terminal kinase (JNK). Accordingly, we observed increased activation of JNK in vivo in cellular and mouse models of Huntington's disease. Additional experiments indicated that the effects of polyQ-Htt on FAT were mediated by neuron-specific JNK3 and not by ubiquitously expressed JNK1, providing a molecular basis for neuron-specific pathology in Huntington's disease. Mass spectrometry identified a residue in the kinesin-1 motor domain that was phosphorylated by JNK3 and this modification reduced kinesin-1 binding to microtubules. These data identify JNK3 as a critical mediator of polyQ-Htt toxicity and provide a molecular basis for polyQ-Htt–induced inhibition of FAT.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Change history
25 June 2009
In the version of this article initially published, a critical word was missing. The sentence should read: "Conversely, antibodies to Htt immunoprecipitated Htt from both wild-type and homozygous HttQ109 knock-in mouse brain lysates, but kinesin-1, KLC, DIC and DHC could not be detected in Htt immunoprecipitates." The error has been corrected in the HTML and PDF versions of the article.
References
Okun, M.S. Huntington's disease: what we learned from the original essay. Neurologist 9, 175–179 (2003).
Orr, H.T. & Zoghbi, H.Y. Trinucleotide repeat disorders. Annu. Rev. Neurosci. 30, 575–621 (2007).
Morfini, G., Pigino, G. & Brady, S.T. Polyglutamine expansion diseases: failing to deliver. Trends Mol. Med. 11, 64–70 (2005).
Gunawardena, S. et al. Disruption of axonal transport by loss of huntingtin or expression of pathogenic polyQ proteins in Drosophila. Neuron 40, 25–40 (2003).
Lee, W.C., Yoshihara, M. & Littleton, J.T. Cytoplasmic aggregates trap polyglutamine-containing proteins and block axonal transport in a Drosophila model of Huntington's disease. Proc. Natl. Acad. Sci. USA 101, 3224–3229 (2004).
Trushina, E. et al. Mutant huntingtin impairs axonal trafficking in mammalian neurons in vivo and in vitro. Mol. Cell. Biol. 24, 8195–8209 (2004).
Morfini, G. et al. JNK mediates pathogenic effects of polyglutamine-expanded androgen receptor on fast axonal transport. Nat. Neurosci. 9, 907–916 (2006).
Szebenyi, G. et al. Neuropathogenic forms of huntingtin and androgen receptor inhibit fast axonal transport. Neuron 40, 41–52 (2003).
Colin, E. et al. Huntingtin phosphorylation acts as a molecular switch for anterograde/retrograde transport in neurons. EMBO J. 27, 2124–2134 (2008).
Gauthier, L.R. et al. Huntingtin controls neurotrophic support and survival of neurons by enhancing BDNF vesicular transport along microtubules. Cell 118, 127–138 (2004).
Engelender, S. et al. Huntingtin-associated protein 1 (HAP1) interacts with the p150Glued subunit of dynactin. Hum. Mol. Genet. 6, 2205–2212 (1997).
Caviston, J.P., Ross, J.L., Antony, S.M., Tokito, M. & Holzbaur, E.L. Huntingtin facilitates dynein/dynactin-mediated vesicle transport. Proc. Natl. Acad. Sci. USA 104, 10045–10050 (2007).
DeBoer, S.R. et al. Conventional kinesin holoenzymes are composed of heavy and light chain homodimers. Biochemistry 47, 4535–4543 (2008).
Lazarov, O. et al. Axonal transport, amyloid precursor protein, kinesin-1 and the processing apparatus: revisited. J. Neurosci. 25, 2386–2395 (2005).
Wheeler, V.C. et al. Long glutamine tracts cause nuclear localization of a novel form of huntingtin in medium spiny striatal neurons in HdhQ92 and HdhQ111 knock-in mice. Hum. Mol. Genet. 9, 503–513 (2000).
Brill, L.B. II & Pfister, K.K. Biochemical and molecular analysis of the mammalian cytoplasmic dynein intermediate chain. Methods 22, 307–316 (2000).
Brady, S.T., Pfister, K.K. & Bloom, G.S. A monoclonal antibody against kinesin inhibits both anterograde and retrograde fast axonal transport in squid axoplasm. Proc. Natl. Acad. Sci. USA 87, 1061–1065 (1990).
Morfini, G., Szebenyi, G., Elluru, R., Ratner, N. & Brady, S.T. Glycogen synthase kinase 3 phosphorylates kinesin light chains and negatively regulates kinesin-based motility. EMBO J. 21, 281–293 (2002).
Donelan, M.J. et al. Ca2+-dependent dephosphorylation of kinesin heavy chain on beta-granules in pancreatic beta-cells. Implications for regulated beta-granule transport and insulin exocytosis. J. Biol. Chem. 277, 24232–24242 (2002).
Apostol, B.L. et al. Mutant huntingtin alters MAPK signaling pathways in PC12 and striatal cells: ERK1/2 protects against mutant huntingtin-associated toxicity. Hum. Mol. Genet. 15, 273–285 (2006).
Merienne, K., Helmlinger, D., Perkin, G.R., Devys, D. & Trottier, Y. Polyglutamine expansion induces a protein-damaging stress connecting heat shock protein 70 to the JNK pathway. J. Biol. Chem. 278, 16957–16967 (2003).
Liu, Y.F. Expression of polyglutamine-expanded Huntingtin activates the SEK1-JNK pathway and induces apoptosis in a hippocampal neuronal cell line. J. Biol. Chem. 273, 28873–28877 (1998).
Coffey, E.T. et al. c-Jun N-terminal protein kinase (JNK) 2/3 is specifically activated by stress, mediating c-Jun activation, in the presence of constitutive JNK1 activity in cerebellar neurons. J. Neurosci. 22, 4335–4345 (2002).
Fabian, M.A. et al. A small molecule-kinase interaction map for clinical kinase inhibitors. Nat. Biotechnol. 23, 329–336 (2005).
Barr, R.K., Kendrick, T.S. & Bogoyevitch, M.A. Identification of the critical features of a small peptide inhibitor of JNK activity. J. Biol. Chem. 277, 10987–10997 (2002).
Dompierre, J.P. et al. Histone deacetylase 6 inhibition compensates for the transport deficit in Huntington's disease by increasing tubulin acetylation. J. Neurosci. 27, 3571–3583 (2007).
Kozikowski, A.P. et al. Functional differences in epigenetic modulators-superiority of mercaptoacetamide-based histone deacetylase inhibitors relative to hydroxamates in cortical neuron neuroprotection studies. J. Med. Chem. 50, 3054–3061 (2007).
Gallo, K.A. & Johnson, G.L. Mixed-lineage kinase control of JNK and p38 MAPK pathways. Nat. Rev. Mol. Cell Biol. 3, 663–672 (2002).
Qin, Z.H. et al. Huntingtin bodies sequester vesicle-associated proteins by a polyproline-dependent interaction. J. Neurosci. 24, 269–281 (2004).
Björkblom, B. et al. All JNKs can kill, but nuclear localization is critical for neuronal death. J. Biol. Chem. 283, 19704–19713 (2008).
Nishitoh, H. et al. ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev. 16, 1345–1355 (2002).
Coffey, E.T., Hongisto, V., Dickens, M., Davis, R.J. & Courtney, M.J. Dual roles for c-Jun N-terminal kinase in developmental and stress responses in cerebellar granule neurons. J. Neurosci. 20, 7602–7613 (2000).
Thomas, G.M., Lin, D.T., Nuriya, M. & Huganir, R.L. Rapid and bi-directional regulation of AMPA receptor phosphorylation and trafficking by JNK. EMBO J. 27, 361–372 (2008).
Ito, M. et al. Isoforms of JSAP1 scaffold protein generated through alternative splicing. Gene 255, 229–234 (2000).
Nühse, T.S., Stensballe, A., Jensen, O.N. & Peck, S.C. Large-scale analysis of in vivo phosphorylated membrane proteins by immobilized metal ion affinity chromatography and mass spectrometry. Mol. Cell. Proteomics 2, 1234–1243 (2003).
Sack, S. et al. X-ray structure of motor and neck domains from rat brain kinesin. Biochemistry 36, 16155–16165 (1997).
Jacobson, C., Schnapp, B. & Banker, G.A. A change in the selective translocation of the kinesin-1 motor domain marks the initial specification of the axon. Neuron 49, 797–804 (2006).
LaPointe, N.E. et al. The amino terminus of tau inhibits kinesin-dependent axonal transport: implications for filament toxicity. J. Neurosci. Res. 87, 440–451 (2009).
Pigino, G., Morfini, G., Mattson, M.P., Brady, S.T. & Busciglio, J. Alzheimer's presenilin 1 mutations impair kinesin-based axonal transport. J. Neurosci. 23, 4499–4508 (2003).
Roy, S., Zhang, B., Lee, V.M. & Trojanowski, J.Q. Axonal transport defects: a common theme in neurodegenerative diseases. Acta Neuropathol. 109, 5–13 (2005).
McGuire, J.R., Rong, J., Li, S.H. & Li, X.J. Interaction of Huntingtin-associated protein-1 with kinesin light chain: implications in intracellular trafficking in neurons. J. Biol. Chem. 281, 3552–3559 (2006).
Li, H., Li, S.H., Yu, Z.X., Shelbourne, P. & Li, X.J. Huntingtin aggregate–associated axonal degeneration is an early pathological event in Huntington's disease mice. J. Neurosci. 21, 8473–8481 (2001).
Carmichael, J., Sugars, K.L., Bao, Y.P. & Rubinsztein, D.C. Glycogen synthase kinase–3beta inhibitors prevent cellular polyglutamine toxicity caused by the Huntington's disease mutation. J. Biol. Chem. 277, 33791–33798 (2002).
Colin, E. et al. Akt is altered in an animal model of Huntington's disease and in patients. Eur. J. Neurosci. 21, 1478–1488 (2005).
Garcia, M., Charvin, D. & Caboche, J. Expanded huntingtin activates the c-Jun terminal kinase/c-Jun pathway prior to aggregate formation in striatal neurons in culture. Neuroscience 127, 859–870 (2004).
Gupta, S. et al. Selective interaction of JNK protein kinase isoforms with transcription factors. EMBO J. 15, 2760–2770 (1996).
Yang, D.D. et al. Absence of excitotoxicity-induced apoptosis in the hippocampus of mice lacking the Jnk3 gene. Nature 389, 865–870 (1997).
Bogoyevitch, M.A. & Kobe, B. Uses for JNK: the many and varied substrates of the c-Jun N-terminal kinases. Microbiol. Mol. Biol. Rev. 70, 1061–1095 (2006).
Cha, J.H. Transcriptional signatures in Huntington's disease. Prog. Neurobiol. 83, 228–248 (2007).
Han, D.K., Eng, J., Zhou, H. & Aebersold, R. Quantitative profiling of differentiation-induced microsomal proteins using isotope-coded affinity tags and mass spectrometry. Nat. Biotechnol. 19, 946–951 (2001).
Acknowledgements
We would like to thank M. MacDonald and M. DiFiglia for knock-in mice and huntingtin constructs, respectively, and B. Wang for excellent technical assistance. This work was supported by a 2007/2008 Marine Biological Laboratory summer fellowship to G.A.M., an Huntington's Disease Society of America grant to G.A.M., US National Institutes of Health grants MH066179 to G.B., and Amyotropic Lateral Sclerosis Association, Muscular Dystrophy Association and US National Institutes of Health (NS23868, NS23320, NS41170) grants to S.T.B.
Author information
Authors and Affiliations
Contributions
G.A.M. and S.T.B. carried out the transport and biochemistry experiments and wrote the manuscript. Y.-M.Y., S.L.P., A.K. and K.L. performed transport and biochemistry experiments. K.Y. provided and characterized recombinant JIP. B.B., E.T.C., C.B. and D.H. carried out the mass spectrometry studies. C.-F.H. and G.B. performed the GFP-kinesin experiments. G.P. performed axonal transport and biochemistry experiments. All of the authors reviewed and edited the manuscript.
Corresponding authors
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 (PDF 2782 kb)
Rights and permissions
About this article
Cite this article
Morfini, G., You, YM., Pollema, S. et al. Pathogenic huntingtin inhibits fast axonal transport by activating JNK3 and phosphorylating kinesin. Nat Neurosci 12, 864–871 (2009). https://doi.org/10.1038/nn.2346
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2346
This article is cited by
-
An RNA-targeting CRISPR–Cas13d system alleviates disease-related phenotypes in Huntington’s disease models
Nature Neuroscience (2023)
-
Normal levels of KIF5 but reduced KLC1 levels in both Alzheimer disease and Alzheimer disease in Down syndrome: evidence suggesting defects in anterograde transport
Alzheimer's Research & Therapy (2021)
-
Zebrafish an experimental model of Huntington’s disease: molecular aspects, therapeutic targets and current challenges
Molecular Biology Reports (2021)
-
Excess Rab4 rescues synaptic and behavioral dysfunction caused by defective HTT-Rab4 axonal transport in Huntington’s disease
Acta Neuropathologica Communications (2020)
-
Structural characterization of the RH1-LZI tandem of JIP3/4 highlights RH1 domains as a cytoskeletal motor-binding motif
Scientific Reports (2019)