Abstract
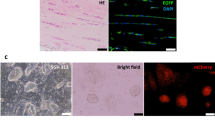
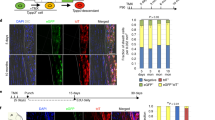
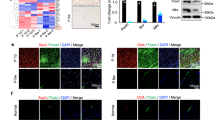
The repair of injured tendons remains a great challenge, largely owing to a lack of in-depth characterization of tendon cells and their precursors. We show that human and mouse tendons harbor a unique cell population, termed tendon stem/progenitor cells (TSPCs), that has universal stem cell characteristics such as clonogenicity, multipotency and self-renewal capacity. The isolated TSPCs could regenerate tendon-like tissues after extended expansion in vitro and transplantation in vivo. Moreover, we show that TSPCs reside within a unique niche predominantly comprised of an extracellular matrix, and we identify biglycan (Bgn) and fibromodulin (Fmod) as two critical components that organize this niche. Depletion of Bgn and Fmod affects the differentiation of TSPCs by modulating bone morphogenetic protein signaling and impairs tendon formation in vivo. Our results, while offering new insights into the biology of tendon cells, may assist in future strategies to treat tendon diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Sharma, P. & Maffulli, N. Biology of tendon injury: healing, modeling and remodeling. J. Musculoskelet. Neuronal Interact. 6, 181–190 (2006).
Kannus, P. Structure of the tendon connective tissue. Scand. J. Med. Sci. Sports 10, 312–320 (2000).
Yoon, J.H. & Halper, J. Tendon proteoglycans: biochemistry and function. J. Musculoskelet. Neuronal Interact. 5, 22–34 (2005).
Fenwick, S. et al. Endochondral ossification in Achilles and patella tendinopathy. Rheumatology (Oxford) 41, 474–476 (2002).
Salingcarnboriboon, R. et al. Establishment of tendon-derived cell lines exhibiting pluripotent mesenchymal stem cell-like property. Exp. Cell Res. 287, 289–300 (2003).
de Mos, M. et al. Intrinsic differentiation potential of adolescent human tendon tissue: an in vitro cell differentiation study. BMC Musculoskelet. Disord. 8, 16 (2007).
Seo, B.M. et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 364, 149–155 (2004).
Fuchs, E., Tumbar, T. & Guasch, G. Socializing with the neighbors: stem cells and their niche. Cell 116, 769–778 (2004).
Taichman, R.S. & Emerson, S.G. Human osteoblasts support hematopoiesis through the production of granulocyte colony-stimulating factor. J. Exp. Med. 179, 1677–1682 (1994).
Zhang, J. et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature 425, 836–841 (2003).
Calvi, L.M. et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 425, 841–846 (2003).
Shen, Q. et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science 304, 1338–1340 (2004).
Shi, S. & Gronthos, S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J. Bone Miner. Res. 18, 696–704 (2003).
Doherty, M.J. et al. Vascular pericytes express osteogenic potential in vitro and in vivo. J. Bone Miner. Res. 13, 828–838 (1998).
Brent, A.E., Schweitzer, R. & Tabin, C.J. A somitic compartment of tendon progenitors. Cell 113, 235–248 (2003).
DiCesare, P.E., Morgelin, M., Mann, K. & Paulsson, M. Cartilage oligomeric matrix protein and thrombospondin 1. Purification from articular cartilage, electron microscopic structure, and chondrocyte binding. Eur. J. Biochem. 223, 927–937 (1994).
Brandau, O., Meindl, A., Fassler, R. & Aszodi, A. A novel gene, tendin, is strongly expressed in tendons and ligaments and shows high homology with chondromodulin-I. Dev. Dyn. 221, 72–80 (2001).
Spangrude, G.J., Heimfeld, S. & Weissman, I.L. Purification and characterization of mouse hematopoietic stem cells. Science 241, 58–62 (1988).
Van Vlasselaer, P., Falla, N., Snoeck, H. & Mathieu, E. Characterization and purification of osteogenic cells from murine bone marrow by two-color cell sorting using anti–Sca-1 monoclonal antibody and wheat germ agglutinin. Blood 84, 753–763 (1994).
Gussoni, E. et al. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature 401, 390–394 (1999).
Tamaki, T. et al. Identification of myogenic-endothelial progenitor cells in the interstitial spaces of skeletal muscle. J. Cell Biol. 157, 571–577 (2002).
Welm, B.E. et al. Sca-1(pos) cells in the mouse mammary gland represent an enriched progenitor cell population. Dev. Biol. 245, 42–56 (2002).
Miura, Y. et al. Defective osteogenesis of the stromal stem cells predisposes CD18-null mice to osteoporosis. Proc. Natl. Acad. Sci. USA 102, 14022–14027 (2005).
Simmons, P.J. & Torok-Storb, B. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood 78, 55–62 (1991).
Filshie, R.J. et al. MUC18, a member of the immunoglobulin superfamily, is expressed on bone marrow fibroblasts and a subset of hematological malignancies. Leukemia 12, 414–421 (1998).
Kuznetsov, S.A. et al. Single-colony derived strains of human marrow stromal fibroblasts form bone after transplantation in vivo. J. Bone Miner. Res. 12, 1335–1347 (1997).
Bi, Y. et al. Extracellular matrix proteoglycans control the fate of bone marrow stromal cells. J. Biol. Chem. 280, 30481–30489 (2005).
Krebsbach, P.H. et al. Bone formation in vivo: comparison of osteogenesis by transplanted mouse and human marrow stromal fibroblasts. Transplantation 63, 1059–1069 (1997).
Bickenbach, J.R. Identification and behavior of label-retaining cells in oral mucosa and skin. J. Dent. Res. 60, 1611–1620 (1981).
Ameye, L. et al. Abnormal collagen fibrils in tendons of biglycan/fibromodulin-deficient mice lead to gait impairment, ectopic ossification, and osteoarthritis. FASEB J. 16, 673–680 (2002).
Cotsarelis, G., Sun, T.T. & Lavker, R.M. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell 61, 1329–1337 (1990).
Morris, R.J. & Potten, C.S. Slowly cycling (label-retaining) epidermal cells behave like clonogenic stem cells in vitro. Cell Prolif. 27, 279–289 (1994).
Booth, C. & Potten, C.S. Gut instincts: thoughts on intestinal epithelial stem cells. J. Clin. Invest. 105, 1493–1499 (2000).
Scadden, D.T. The stem-cell niche as an entity of action. Nature 441, 1075–1079 (2006).
Krause, D.S. Regulation of hematopoietic stem cell fate. Oncogene 21, 3262–3269 (2002).
Arai, F. et al. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell 118, 149–161 (2004).
Moore, K.A. & Lemischka, I.R. Stem cells and their niches. Science 311, 1880–1885 (2006).
Blanpain, C. & Fuchs, E. Epidermal stem cells of the skin. Annu. Rev. Cell Dev. Biol. 22, 339–373 (2006).
Garcion, E., Halilagic, A., Faissner, A. & Ffrench-Constant, C. Generation of an environmental niche for neural stem cell development by the extracellular matrix molecule tenascin C. Development 131, 3423–3432 (2004).
Nilsson, S.K. et al. Osteopontin, a key component of the hematopoietic stem cell niche and regulator of primitive hematopoietic progenitor cells. Blood 106, 1232–1239 (2005).
Ohta, M., Sakai, T., Saga, Y., Aizawa, S. & Saito, M. Suppression of hematopoietic activity in tenascin-C–deficient mice. Blood 91, 4074–4083 (1998).
Stier, S. et al. Osteopontin is a hematopoietic stem cell niche component that negatively regulates stem cell pool size. J. Exp. Med. 201, 1781–1791 (2005).
Schweitzer, R. et al. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development 128, 3855–3866 (2001).
Awad, H.A. et al. Autologous mesenchymal stem cell–mediated repair of tendon. Tissue Eng. 5, 267–277 (1999).
Hoffmann, A. et al. Neotendon formation induced by manipulation of the Smad8 signalling pathway in mesenchymal stem cells. J. Clin. Invest. 116, 940–952 (2006).
Kuznetsov, S.A., Friedenstein, A.J. & Robey, P.G. Factors required for bone marrow stromal fibroblast colony formation in vitro. Br. J. Haematol. 97, 561–570 (1997).
Gimble, J.M. et al. Bone morphogenetic proteins inhibit adipocyte differentiation by bone marrow stromal cells. J. Cell. Biochem. 58, 393–402 (1995).
Johnstone, B., Hering, T.M., Caplan, A.I., Goldberg, V.M. & Yoo, J.U. In vitro chondrogenesis of bone marrow–derived mesenchymal progenitor cells. Exp. Cell Res. 238, 265–272 (1998).
Kostenuik, P.J., Halloran, B.P., Morey-Holton, E.R. & Bikle, D.D. Skeletal unloading inhibits the in vitro proliferation and differentiation of rat osteoprogenitor cells. Am. J. Physiol. 273, E1133–E1139 (1997).
Lopez-Rovira, T., Chalaux, E., Massague, J., Rosa, J.L. & Ventura, F. Direct binding of Smad1 and Smad4 to two distinct motifs mediates bone morphogenetic protein–specific transcriptional activation of Id1 gene. J. Biol. Chem. 277, 3176–3185 (2002).
Acknowledgements
This research was supported in part by the Division of Intramural Research, US National Institute of Dental and Craniofacial Research, US National Institutes of Health and by an extramural grant from the US National Institutes of Health (US National Heart, Lung, and Blood Institute R01 HL61589-01 for L.Z.). We thank Å. Oldberg, University of Lund, Sweden for providing Fmod-deficient mice; P. Robey for advice and discussion on this work; H. Wimer, N. Marino, S. Kuznetsov and N. Cherman for technical assistance; and P. Vyomesh for his help with the isolation of mouse dermal fibroblasts.
Author information
Authors and Affiliations
Contributions
Y.B. designed and performed the majority of the experiments, analyzed data and prepared the manuscript. D.E. performed, collected and analyzed the FACS data; T.M.K. maintained animals, assisted with in vivo experiments and collected tissue samples; C.A.I. performed nucleofection and luciferase reporter assays; M.C.E. and W.S. assisted with immunohistochemistry staining and L.L. prepared paraffin-embedded tissue sections. A.I.L. provided human samples. B.-M.S. helped with in vitro multipotent differentiation assays and in vivo transplantation. L.Z. designed the FACS analysis and helped with the preparation of the manuscript. S.S. designed the key experiments and prepared the manuscript. M.F.Y. performed RT-PCR and prepared the manuscript.
Corresponding authors
Supplementary information
Supplementary Text and Figures
Supplementary Figs. 1–5, Supplementary Methods (PDF 851 kb)
Rights and permissions
About this article
Cite this article
Bi, Y., Ehirchiou, D., Kilts, T. et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med 13, 1219–1227 (2007). https://doi.org/10.1038/nm1630
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1630
This article is cited by
-
In-situ gelation of fibrin gel encapsulating platelet-rich plasma-derived exosomes promotes rotator cuff healing
Communications Biology (2024)
-
Stem Cells and Regenerative Strategies for Wound Healing: Therapeutic and Clinical Implications
Current Pharmacology Reports (2024)
-
Neonatal Achilles Tendon Microstructure is Negatively Impacted by Decorin and Biglycan Knockdown After Injury and During Development
Annals of Biomedical Engineering (2024)
-
Tendon stem/progenitor cells are promising reparative cell sources for multiple musculoskeletal injuries of concomitant articular cartilage lesions associated with ligament injuries
Journal of Orthopaedic Surgery and Research (2023)
-
Caveolin-1 is involved in fatty infiltration and bone-tendon healing of rotator cuff tear
Molecular Medicine (2023)