Abstract
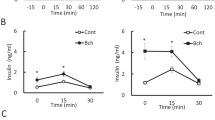
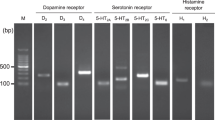
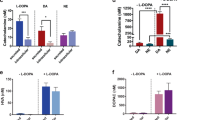
In the nervous system, NMDA receptors (NMDARs) participate in neurotransmission and modulate the viability of neurons. In contrast, little is known about the role of NMDARs in pancreatic islets and the insulin-secreting beta cells whose functional impairment contributes to diabetes mellitus. Here we found that inhibition of NMDARs in mouse and human islets enhanced their glucose-stimulated insulin secretion (GSIS) and survival of islet cells. Further, NMDAR inhibition prolonged the amount of time that glucose-stimulated beta cells spent in a depolarized state with high cytosolic Ca2+ concentrations. We also noticed that, in vivo, the NMDAR antagonist dextromethorphan (DXM) enhanced glucose tolerance in mice, and that in vitro dextrorphan, the main metabolite of DXM, amplified the stimulatory effect of exendin-4 on GSIS. In a mouse model of type 2 diabetes mellitus (T2DM), long-term treatment with DXM improved islet insulin content, islet cell mass and blood glucose control. Further, in a small clinical trial we found that individuals with T2DM treated with DXM showed enhanced serum insulin concentrations and glucose tolerance. Our data highlight the possibility that antagonists of NMDARs may provide a useful adjunct treatment for diabetes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Polonsky, K.S. The past 200 years in diabetes. N. Engl. J. Med. 367, 1332–1340 (2012).
Kahn, S.E., Hull, R.L. & Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444, 840–846 (2006).
Cavaghan, M.K., Ehrmann, D.A. & Polonsky, K.S. Interactions between insulin resistance and insulin secretion in the development of glucose intolerance. J. Clin. Invest. 106, 329–333 (2000).
Ferrannini, E. The stunned beta cell: a brief history. Cell Metab. 11, 349–352 (2010).
Rorsman, P. & Braun, M. Regulation of insulin secretion in human pancreatic islets. Annu. Rev. Physiol. 75, 155–179 (2013).
Talchai, C., Xuan, S., Lin, H.V., Sussel, L. & Accili, D. Pancreatic beta cell dedifferentiation as a mechanism of diabetic beta cell failure. Cell 150, 1223–1234 (2012).
Tahrani, A.A., Bailey, C.J., Del Prato, S. & Barnett, A.H. Management of type 2 diabetes: new and future developments in treatment. Lancet 378, 182–197 (2011).
DeFronzo, R.A. Overview of newer agents: where treatment is going. Am. J. Med. 123, S38–S48 (2010).
Amiel, S.A., Dixon, T., Mann, R. & Jameson, K. Hypoglycaemia in type 2 diabetes. Diabet. Med. 25, 245–254 (2008).
Cryer, P.E. Mechanisms of hypoglycemia-associated autonomic failure in diabetes. N. Engl. J. Med. 369, 362–372 (2013).
Kahn, S.E., Cooper, M.E. & Del Prato, S. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet 383, 1068–1083 (2014).
Retnakaran, R., Kramer, C.K., Choi, H., Swaminathan, B. & Zinman, B. Liraglutide and the preservation of pancreatic beta-cell function in early type 2 diabetes: the LIBRA trial. Diabetes Care 37, 3270–3278 (2014).
Jain, R. et al. Pharmacological inhibition of Eph receptors enhances glucose-stimulated insulin secretion from mouse and human pancreatic islets. Diabetologia 56, 1350–1355 (2013).
Burris, R.E. & Hebrok, M. Pancreatic innervation in mouse development and beta-cell regeneration. Neuroscience 150, 592–602 (2007).
Rodriguez-Diaz, R. & Caicedo, A. Novel approaches to studying the role of innervation in the biology of pancreatic islets. Endocrinol. Metab. Clin. North Am. 42, 39–56 (2013).
Soltani, N. et al. GABA exerts protective and regenerative effects on islet beta cells and reverses diabetes. Proc. Natl. Acad. Sci. USA 108, 11692–11697 (2011).
Rodriguez-Diaz, R. et al. Real-time detection of acetylcholine release from the human endocrine pancreas. Nat. Protoc. 7, 1015–1023 (2012).
Maechler, P. & Wollheim, C.B. Mitochondrial glutamate acts as a messenger in glucose-induced insulin exocytosis. Nature 402, 685–689 (1999).
Cabrera, O. et al. Glutamate is a positive autocrine signal for glucagon release. Cell Metab. 7, 545–554 (2008).
Di Cairano, E.S. et al. The glial glutamate transporter 1 (GLT1) is expressed by pancreatic beta-cells and prevents glutamate-induced beta-cell death. J. Biol. Chem. 286, 14007–14018 (2011).
Feldmann, N. et al. Reduction of plasma membrane glutamate transport potentiates insulin but not glucagon secretion in pancreatic islet cells. Mol. Cell. Endocrinol. 338, 46–57 (2011).
Inagaki, N. et al. Expression and role of ionotropic glutamate receptors in pancreatic islet cells. FASEB 9, 686–691 (1995).
Garnock-Jones, K.P. Dextromethorphan/quinidine: in pseudobulbar affect. CNS Drugs 25, 435–445 (2011).
Hamosh, A., Maher, J.F., Bellus, G.A., Rasmussen, S.A. & Johnston, M.V. Long-term use of high-dose benzoate and dextromethorphan for the treatment of nonketotic hyperglycinemia. J. Pediatr. 132, 709–713 (1998).
Nelson, K.A., Park, K.M., Robinovitz, E., Tsigos, C. & Max, M.B. High-dose oral dextromethorphan versus placebo in painful diabetic neuropathy and postherpetic neuralgia. Neurology 48, 1212–1218 (1997).
Pechnick, R.N. & Poland, R.E. Comparison of the effects of dextromethorphan, dextrorphan, and levorphanol on the hypothalamo-pituitary-adrenal axis. J. Pharmacol. Exp. Ther. 309, 515–522 (2004).
Yashiro, K. & Philpot, B.D. Regulation of NMDA receptor subunit expression and its implications for LTD, LTP, and metaplasticity. Neuropharmacology 55, 1081–1094 (2008).
Lee, C.H. et al. NMDA receptor structures reveal subunit arrangement and pore architecture. Nature 511, 191–197 (2014).
Kalia, L.V., Kalia, S.K. & Salter, M.W. NMDA receptors in clinical neurology: excitatory times ahead. Lancet Neurol. 7, 742–755 (2008).
Ngo-Anh, T.J. et al. SK channels and NMDA receptors form a Ca2+-mediated feedback loop in dendritic spines. Nat. Neurosci. 8, 642–649 (2005).
Isaacson, J.S. & Murphy, G.J. Glutamate-mediated extrasynaptic inhibition: direct coupling of NMDA receptors to Ca(2+)-activated K+ channels. Neuron 31, 1027–1034 (2001).
Hardingham, G.E. & Bading, H. Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat. Rev. Neurosci. 11, 682–696 (2010).
Bartlett, T.E. & Wang, Y.T. The intersections of NMDAR-dependent synaptic plasticity and cell survival. Neuropharmacology 74, 59–68 (2013).
Rodríguez-Moreno, A., Banerjee, A. & Paulsen, O. Presynaptic NMDA receptors and spike timing-dependent depression at cortical synapses. Front. Synaptic Neurosci. 2, 18 (2010).
Gonoi, T. et al. Functional neuronal ionotropic glutamate receptors are expressed in the non-neuronal cell line MIN6. J. Biol. Chem. 269, 16989–16992 (1994).
Molnár, E., Varadi, A., McIlhinney, R.A. & Ashcroft, S.J. Identification of functional ionotropic glutamate receptor proteins in pancreatic beta-cells and in islets of Langerhans. FEBS Lett. 371, 253–257 (1995).
Garrino, M.G. & Henquin, J.C. Adamantane derivatives: a new class of insulin secretagogues. Br. J. Pharmacol. 90, 583–591 (1987).
Konrad, D., Sobetzko, D., Schmitt, B. & Schoenle, E.J. Insulin-dependent diabetes mellitus induced by the antitussive agent dextromethorphan. Diabetologia 43, 261–262 (2000).
Lechin, F. et al. Amantadine reduces glucagon and enhances insulin secretion throughout the oral glucose tolerance test: central plus peripheral nervous system mechanisms. Diabetes Metab. Syndr. Obes. 2, 203–213 (2009).
Ashcroft, F.M., Kerr, A.J., Gibson, J.S. & Williams, B.A. Amantadine and sparteine inhibit ATP-regulated K-currents in the insulin-secreting beta-cell line, HIT-T15. Br. J. Pharmacol. 104, 579–584 (1991).
Wong, E.H.F. et al. The anticonvulsant Mk-801 is a potent N-methyl-D-aspartate Antagonist. Proc. Natl. Acad. Sci. USA 83, 7104–7108 (1986).
Li, L. & Hanahan, D. Hijacking the neuronal NMDAR signaling circuit to promote tumor growth and invasion. Cell 153, 86–100 (2013).
Olney, J.W., Labruyere, J. & Price, M.T. Pathological changes induced in cerebrocortical neurons by phencyclidine and related drugs. Science 244, 1360–1362 (1989).
Gheni, G. et al. Glutamate acts as a key signal linking glucose metabolism to incretin/cAMP action to amplify insulin secretion. Cell Reports 9, 661–673 (2014).
Speier, S. & Rupnik, M. A novel approach to in situ characterization of pancreatic beta-cells. Pflugers Arch. 446, 553–558 (2003).
Miki, T. et al. Defective insulin secretion and enhanced insulin action in KATP channel-deficient mice. Proc. Natl. Acad. Sci. USA 95, 10402–10406 (1998).
Begenisich, T. et al. Physiological roles of the intermediate conductance, Ca2+-activated potassium channel Kcnn4. J. Biol. Chem. 279, 47681–47687 (2004).
Düfer, M. et al. Enhanced glucose tolerance by SK4 channel inhibition in pancreatic beta-cells. Diabetes 58, 1835–1843 (2009).
Gilon, P., Shepherd, R.M. & Henquin, J.C. Oscillations of secretion driven by oscillations of cytoplasmic Ca2+ as evidences in single pancreatic islets. J. Biol. Chem. 268, 22265–22268 (1993).
Bergsten, P., Grapengiesser, E., Gylfe, E., Tengholm, A. & Hellman, B. Synchronous oscillations of cytoplasmic Ca2+ and insulin release in glucose-stimulated pancreatic islets. J. Biol. Chem. 269, 8749–8753 (1994).
Bardy, G. et al. Quercetin induces insulin secretion by direct activation of L-type calcium channels in pancreatic beta cells. Br. J. Pharmacol. 169, 1102–1113 (2013).
Dadi, P.K. et al. Inhibition of pancreatic beta-cell Ca2+/calmodulin-dependent protein kinase II reduces glucose-stimulated calcium influx and insulin secretion, impairing glucose tolerance. J. Biol. Chem. 289, 12435–12445 (2014).
Dixit, S.S. et al. Effects of CaMKII-mediated phosphorylation of ryanodine receptor type 2 on islet calcium handling, insulin secretion, and glucose tolerance. PLoS ONE 8, e58655 (2013).
Maurice, T., Urani, A., Phan, V.L. & Romieu, P. The interaction between neuroactive steroids and the sigma1 receptor function: behavioral consequences and therapeutic opportunities. Brain Res. Brain Res. Rev. 37, 116–132 (2001).
Drucker, D.J. & Nauck, M.A. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 368, 1696–1705 (2006).
Coleman, D.L. & Hummel, K.P. Studies with the mutation, diabetes, in the mouse. Diabetologia 3, 238–248 (1967).
Donath, M.Y. Targeting inflammation in the treatment of type 2 diabetes: time to start. Nat. Rev. Drug Discov. 13, 465–476 (2014).
Ashcroft, F.M. & Rorsman, P. Electrophysiology of the pancreatic beta-cell. Prog. Biophys. Mol. Biol. 54, 87–143 (1989).
Shen, K.Z. & Johnson, S.W. Ca2+ influx through NMDA-gated channels activates ATP-sensitive K+ currents through a nitric oxide-cGMP pathway in subthalamic neurons. J. Neurosci. 30, 1882–1893 (2010).
Schiemann, J. et al. K-ATP channels in dopamine substantia nigra neurons control bursting and novelty-induced exploration. Nat. Neurosci. 15, 1272–1280 (2012).
Gu, G., Dubauskaite, J. & Melton, D.A. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development 129, 2447–2457 (2002).
Thorens, B. et al. Ins1Cre knock-in mice for beta cell-specific gene recombination. Diabetologia 58, 558–565 (2015).
Yesil, P. et al. A new collagenase blend increases the number of islets isolated from mouse pancreas. Islets 1, 185–190 (2009).
Janjic, D. et al. Free radical modulation of insulin release in INS-1 cells exposed to alloxan. Biochem. Pharmacol. 57, 639–648 (1999).
Kopec, C.D., Li, B., Wei, W., Boehm, J. & Malinow, R. Glutamate receptor exocytosis and spine enlargement during chemically induced long-term potentiation. J. Neurosci. 26, 2000–2009 (2006).
Silva, J.P. et al. Impaired insulin secretion and beta-cell loss in tissue-specific knockout mice with mitochondrial diabetes. Nat. Genet. 26, 336–340 (2000).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Berggren, P.O. et al. Removal of Ca2+ channel beta3 subunit enhances Ca2+ oscillation frequency and insulin exocytosis. Cell 119, 273–284 (2004).
Stožer, A., Dolensek, J. & Rupnik, M.S. Glucose-stimulated calcium dynamics in islets of Langerhans in acute mouse pancreas tissue slices. PLoS ONE 8, e54638 (2013).
Loos, W.J. et al. Simultaneous quantification of dextromethorphan and its metabolites dextrorphan, 3-methoxymorphinan and 3-hydroxymorphinan in human plasma by ultra performance liquid chromatography/tandem triple-quadrupole mass spectrometry. J. Pharm. Biomed. Anal. 54, 387–394 (2011).
Acknowledgements
We thank C.B. Wollheim and P. Maechler (University of Geneva) for providing INS1E cells, J. Roeper (Goethe University Frankfurt) and S. Seino (Kobe University) for providing Kir6.2-deficient mice, H. Bading (University of Heidelberg) and his colleagues for providing us with a GluN1-positive control and for helpful discussions, and S. Jakob, B. Bartosinska and A. Leinweber (Heinrich Heine University Düsseldorf) for technical support. We also thank O. Kuß (German Diabetes Center Düsseldorf) and B. Kronhage (Profil Institute for Metabolic Research) for supporting the statistical analyses of the clinical and preclinical data, respectively. P.-O.B. and M. Köhler are supported by the Swedish Research Council and the Family Erling-Persson Foundation. This study was supported by the Heinrich Heine University Düsseldorf; its research training group entitled “In vivo investigations in metabolic pathomechanisms and diseases in Düsseldorf” and its research commission of the medical faculty; the German Center for Diabetes Research; the German Diabetes Center; the Federal Ministry of Health; the Ministry for Innovation, Science and Research of North Rhine–Westphalia; Ipsen Pharma GmbH; and the Anton-Betz Foundation. Research from M.S.K., A. Stožer and M.S.R. was produced within the framework of an operation entitled “Centre of Open Innovation and Research UM (CORE@UM)” (3330-13-500032) and co-funded by the European Regional Development Fund. D.E. and E.L. are supported by the German Research Foundation (La1216/6-1), and M. Kragl and E.L. are supported by the Competence Network Diabetes Mellitus from the Federal Ministry for Education and Research. Human islets were obtained either by L.P. (ECIT consortium, supported by JDRF, Grant Name: Islets for Research; Grant Number: 31-2008-416) or from the NIDDK-funded Integrated Islet Distribution Program (IIDP) at City of Hope. A list of all participating IIDP laboratories can be found here: https://iidp.coh.org/center_info.aspx.
Author information
Authors and Affiliations
Contributions
J.M. performed the initial experiments; J.M., A.W. and, to some extent, S.O. performed insulin secretion assays; J.M., S.O., A.W. performed IPGTTs; J.M. carried out dextromethorphan treatment of db/db mice and performed experiments with INS1E cells; S.O. determined islet cell proliferation, beta cell mass and apoptosis, and performed the western blots and Ca2+ measurements set up by M. Köhler; A.W. performed in vitro islet cell viability experiments, reproduced the effects of dextromethorphan treatment in db/db mice and determined corticosterone concentrations; P.-O.B. introduced the idea of NMDAR-regulated Ca2+ oscillations to E.L., designed and performed the dynamic insulin release measurements, and together with M. Köhler established the technique for Ca2+ measurements in the laboratory of E.L.; M. Kragl and D.E. supervised J.M. and S.O. in standard techniques of islet biology and glucose measurements and helped with mouse experiments; J.E. trained S.O. and A.W. in image analysis and contributed to statistical analyses; A.F. drew a statistical analysis for clinical trial design and performed statistical analysis of human and mouse data; A. Stirban, F.S. and T.H. guided the clinical trial whose study protocol was written by A. Stirban with input from F.S., T.H., E.L., J.M. and T.M.; insulin and DXM concentrations from the clinical trial were measured by S.W. and D.H., respectively; J.F. and B.T. generated and analyzed the Ins1-cre mice; T.M. introduced the idea to use DXM for treatment of hyperinsulinism from islets to E.L.; M.S.K., A. Stožer and M.S.R. performed the Ca2+ and membrane potential measurements in pancreatic sections; O.K. and N.K. gave intellectual and experimental input into NMDAR physiology; E.M. co-supervised A.W.; L.P. performed human islet isolation; and E.L. conceptually designed most parts of this work, introduced the idea to use DXM as an antidiabetic compound, genetically study GluN1 as well as test DXM on islet cell viability and in GluN1 deficient mice, db/db mice and human individuals with T2DM, guided J.M., S.O. and A.W. in biweekly meetings, and wrote the article with them.
Corresponding author
Ethics declarations
Competing interests
J.M., T.M. and E.L. pursue a patent application (WO 2013/029762 A1) entitled Morphinan-derivatives for treating diabetes and related disorders.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8, Supplementary Tables 1–2, and Supplementary Note (PDF 5015 kb)
Rights and permissions
About this article
Cite this article
Marquard, J., Otter, S., Welters, A. et al. Characterization of pancreatic NMDA receptors as possible drug targets for diabetes treatment. Nat Med 21, 363–372 (2015). https://doi.org/10.1038/nm.3822
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3822
This article is cited by
-
Cystine/glutamate antiporter System xc- deficiency impairs insulin secretion in mice
Diabetologia (2023)
-
The effect of glycine administration on the characteristics of physiological systems in human adults: A systematic review
GeroScience (2023)
-
Sphingolipid subtypes differentially control proinsulin processing and systemic glucose homeostasis
Nature Cell Biology (2023)
-
NMDA receptor-mediated CaMKII/ERK activation contributes to renal fibrosis
BMC Nephrology (2020)
-
A Study on Insulin Levels and the Expression of Glut 4 in Streptozotocin (STZ) Induced Diabetic Rats Treated with Mustard Oil Diet
Indian Journal of Clinical Biochemistry (2020)