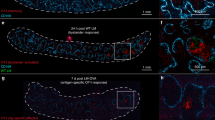
Abstract
The maturation status of dendritic cells (DCs) determines whether they prime or tolerize T cells. We targeted ovalbumin peptide exclusively to DCs in situ using an antibody to DEC-205 and studied the interaction of DCs with naive CD4+ T cells in tolerizing or priming conditions. We used two-photon microscopy to simultaneously track antigen-specific OT-II T cells, nonspecific T cells and DCs in lymph nodes of living mice. In both tolerance and immunity, OT-II cells arrested on DCs near high endothelial venules beginning shortly after extravasation and regained their baseline speed by 18 h. Thus, early antigen-dependent T cell arrest on DCs is a shared feature of tolerance and priming associated with activation and proliferation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Stoll, S., Delon, J., Brotz, T.M. & Germain, R.N. Dynamic imaging of T cell-dendritic cell interactions in lymph nodes. Science 296, 1873–1876 (2002).
Miller, M.J., Wei, S.H., Parker, I. & Cahalan, M.D. Two-photon imaging of lymphocyte motility and antigen response in intact lymph node. Science 296, 1869–1873 (2002).
Bousso, P. & Robey, E. Dynamics of CD8+ T cell priming by dendritic cells in intact lymph nodes. Nat. Immunol. 4, 579–585 (2003).
Miller, M.J., Hejazi, A.S., Wei, S.H., Cahalan, M.D. & Parker, I. T cell repertoire scanning is promoted by dynamic dendritic cell behavior and random T cell motility in the lymph node. Proc. Natl. Acad. Sci. USA 101, 998–1003 (2004).
Miller, M.J., Safrina, O., Parker, I. & Cahalan, M.D. Imaging the single cell dynamics of CD4+ T cell activation by dendritic cells in lymph nodes. J. Exp. Med. 200, 847–856 (2004).
Hugues, S. et al. Distinct T cell dynamics in lymph nodes during the induction of tolerance and immunity. Nat. Immunol. 5, 1235–1242 (2004).
Mempel, T.R., Henrickson, S.E. & Von Andrian, U.H. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature 427, 154–159 (2004).
Miller, M.J., Wei, S.H., Cahalan, M.D. & Parker, I. Autonomous T cell trafficking examined in vivo with intravital two-photon microscopy. Proc. Natl. Acad. Sci. USA 100, 2604–2609 (2003).
Gunzer, M. et al. A spectrum of biophysical interaction modes between T cells and different antigen presenting cells during priming in 3-D collagen and in vivo. Blood 104, 2801–2809 (2004).
Lindquist, R.L. et al. Visualizing dendritic cell networks in vivo. Nat. Immunol. 5, 1243–1250 (2004).
Banchereau, J., Pascual, V. & Palucka, K.A. Autoimunity through cytokine-induced dendritic cell activation. Immunity 20, 539–550 (2004).
Guermonprez, P., Valladeau, J., Zitvogel, L., Thery, C. & Amigorena, S. Antigen presentation and T cell stimulation by dendritic cells. Annu. Rev. Immunol. 20, 621–667 (2002).
Lanzavecchia, A. & Sallusto, F. Regulation of T cell immunity by dendritic cells. Cell 106, 263–266 (2001).
Liu, Y.J. Dendritic cell subsets and lineages, and their functions in innate and adaptive immunity. Cell 106, 259–262 (2001).
Mellman, I. & Steinman, R.M. Dendritic cells: specialized and regulated antigen processing machines. Cell 106, 255–258 (2001).
Steinman, R.M., Hawiger, D. & Nussenzweig, M.C. Tolerogenic dendritic cells. Annu. Rev. Immunol. 21, 685–711 (2003).
Belz, G.T. et al. The CD8α+ dendritic cell is responsible for inducing peripheral self-tolerance to tissue-associated antigens. J. Exp. Med. 196, 1099–1104 (2002).
Huang, F.P. et al. A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes. J. Exp. Med. 191, 435–444 (2000).
Inaba, K. et al. Efficient presentation of phagocytosed cellular fragments on the major histocompatibility complex class II products of dendritic cells. J. Exp. Med. 188, 2163–2173 (1998).
Iyoda, T. et al. The CD8+ dendritic cell subset selectively endocytoses dying cells in culture and in vivo. J. Exp. Med. 195, 1289–1302 (2002).
Liu, K. et al. Immune tolerance after delivery of dying cells to dendritic cells in situ. J. Exp. Med. 196, 1091–1097 (2002).
Scheinecker, C., McHugh, R., Shevach, E.M. & Germain, R.N. Constitutive presentation of a natural tissue autoantigen exclusively by dendritic cells in the draining lymph node. J. Exp. Med. 196, 1079–1090 (2002).
Hawiger, D. et al. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J. Exp. Med. 194, 769–779 (2001).
Bonifaz, L. et al. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J. Exp. Med. 196, 1627–1638 (2002).
Probst, H.C., Lagnel, J., Kollias, G. & van den Broek, M. Inducible transgenic mice reveal resting dendritic cells as potent inducers of CD8+ T cell tolerance. Immunity 18, 713–720 (2003).
Brimnes, M.K., Bonifaz, L., Steinman, R.M. & Moran, T.M. Influenza virus-induced dendritic cell maturation is associated with the induction of strong T cell immunity to a coadministered, normally nonimmunogenic protein. J. Exp. Med. 198, 133–144 (2003).
Hawiger, D., Masilamani, R., Betelli, E., Kuchroo, V.J. & Nussenzweig, M.C. Immunological unresponsiveness characterized by increased expression of CD5 on peripheral T cells induced by dendritic cells in vivo. Immunity 20, 695–705 (2004).
Kurts, C., Kosaka, H., Carbone, F.R., Miller, J.F. & Heath, W.R. Class I-restricted cross-presentation of exogenous self-antigens leads to deletion of autoreactive CD8+ T cells. J. Exp. Med. 186, 239–245 (1997).
Zal, T., Volkmann, A. & Stockinger, B. Mechanisms of tolerance induction in major histocompatibility complex class II-restricted T cells specific for a blood-borne self-antigen. J. Exp. Med. 180, 2089–2099 (1994).
Bonifaz, L.C. et al. In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J. Exp. Med. 199, 815–824 (2004).
Kearney, E.R., Pape, K.A., Loh, D.Y. & Jenkins, M.K. Visualization of peptide-specific T cell immunity and peripheral tolerance induction in vivo. Immunity 1, 327–339 (1994).
Van Parijs, L., Peterson, D.A. & Abbas, A.K. The Fas/Fas ligand pathway and Bcl-2 regulate T cell responses to model self and foreign antigens. Immunity 8, 265–274 (1998).
Redmond, W.L., Hernandez, J. & Sherman, L.A. Deletion of naive CD8 T cells requires persistent antigen and is not programmed by an initial signal from the tolerogenic APC. J. Immunol. 171, 6349–6354 (2003).
Barnden, M.J., Allison, J., Heath, W.R. & Carbone, F.R. Defective TCR expression in transgenic mice constructed using cDNA-based α- and β-chain genes under the control of heterologous regulatory elements. Immunol. Cell Biol. 76, 34–40 (1998).
Huang, A.Y., Qi, H. & Germain, R.N. Illuminating the landscape of in vivo immunity: insights from dynamic in situ imaging of secondary lymphoid tissues. Immunity 21, 331–339 (2004).
Sumen, C., Mempel, T.R., Mazo, I.B. & von Andrian, U.H. Intravital microscopy: visualizing immunity in context. Immunity 21, 315–329 (2004).
Bajenoff, M., Granjeaud, S. & Guerder, S. The strategy of T cell antigen-presenting cell encounter in antigen-draining lymph nodes revealed by imaging of initial T cell activation. J. Exp. Med. 198, 715–724 (2003).
Negulescu, P.A., Krasieva, T.B., Khan, A., Kerschbaum, H.H. & Cahalan, M.D. Polarity of T cell shape, motility, and sensitivity to antigen. Immunity 4, 421–430 (1996).
Dustin, M.L., Bromley, S.K., Kan, Z., Peterson, D.A. & Unanue, E.R. Antigen receptor engagement delivers a stop signal to migrating T lymphocytes. Proc. Natl. Acad. Sci. USA 94, 3909–3913 (1997).
Ohl, L. et al. CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity 21, 279–288 (2004).
Benvenuti, F. et al. Dendritic cell maturation controls adhesion, synapse formation, and the duration of the interactions with naive T lymphocytes. J. Immunol. 172, 292–301 (2004).
Wulfing, C. et al. Costimulation and endogenous MHC ligands contribute to T cell recognition. Nat. Immunol. 3, 42–47 (2002).
Huppa, J.B., Gleimer, M., Sumen, C. & Davis, M.M. Continuous T cell receptor signaling required for synapse maintenance and full effector potential. Nat. Immunol. 4, 749–755 (2003).
Valitutti, S., Muller, S., Cella, M., Padovan, E. & Lanzavecchia, A. Serial triggering of many T-cell receptors by a few peptide-MHC complexes. Nature 375, 148–151 (1995).
Itano, A.A. et al. Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity 19, 47–57 (2003).
Kang, Y.S. et al. SIGN-R1, a novel C-type lectin expressed by marginal zone macrophages in spleen, mediates uptake of the polysaccharide dextran. Int. Immunol. 15, 177–186 (2003).
Wright, D.E. et al. Cyclophosphamide/granulocyte colony-stimulating factor causes selective mobilization of bone marrow hematopoietic stem cells into the blood after M phase of the cell cycle. Blood 97, 2278–2285 (2001).
Hadjantonakis, A.K., Macmaster, S. & Nagy, A. Embryonic stem cells and mice expressing different GFP variants for multiple non-invasive reporter usage within a single animal. BMC Biotechnol. 2, 11 (2002).
Acknowledgements
We thank R. Steinman for discussions; E. Besmer for help with the manuscript; W. Gan for guidance on two-photon microscopy; S. Boscardin for α-DEC-CSP; and R. Masalimani for α-DEC–OVA plasmid. M.C.N. and M.L.D. contributed equally to this work. Supported by the National Institutes of Health (AI055037 to M.L.D. and AI051573 to M.C.N.), Irene Diamond Foundation (M.L.D.), Howard Hughes Medical Institute (M.C.N.), Rothschild Foundation (G.S.), Medical Scientist Training Program (GM07739 to R.L.L.) and German Research Foundation (DU 548/1-1 to D.D.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
DC maturation induced by α-CD40 antibody. (PDF 304 kb)
Supplementary Fig. 2
Timeline for imaging T cell tolerance and priming. (PDF 1002 kb)
Supplementary Fig. 3
There is no systematic association between cell depth and average cell speed. (PDF 529 kb)
Supplementary Fig. 4
Instantaneous velocities of specific and non-specific cells. (PDF 116 kb)
Supplementary Fig. 5
Speeds of antigen-specific cells normalized to speed of control cells in the same imaging field. (PDF 59 kb)
Supplementary Video 1
Two-photon visualization of 3 types of cells and the vasculature in live lymph nodes (MOV 3182 kb)
This and all movies below are maximum intensity projections of a 50 μm-thick volume. Lymph node vasculature can be visualized with Q-dots that emit light at 655 nm. When excited at 910 nm, Q-dots are bright enough to visualize even capillaries, which was not possible with the small molecule-conjugated dextrans. HEVs can be identified by the morphology of the endothelial cells and the shadows of rolling lymphocytes. Antigen-specific EGFP-OT-II T cells (cyan) and non-specific ECFP-T cells (blue) move similarly in this field.
Supplementary Video 2
T cell extravasation in the lymph node of a living mouse (MOV 3160 kb)
T cells (blue, cyan) are visible moving throughout the vasculature (red), visualized by i.v. injection of Q-dots. Many vessels are partially in contact with dendritic cells (yellow). A single T cell is visible extravasating in the lower right quadrant, indicated by an arrow – it appears in the middle of the HEV at 8:11, remains relatively stationary for ten minutes and moves to the left and up over the next ten minutes
Supplementary Video 3
Phototoxicity interferes with dendrite probing and cell migration (MOV 3021 kb)
Even before phototoxicity is manifested in cell morphology, it leads to cellular immobility. Lymphocytes (red) stop moving and DCs (green) stop the probing motions of their dendrites. The use of both dendrite movement and non-specific T cells as internal controls permits sensitive detection of damage to the imaging field. In the upper right corner of the field, which was not affected, both T cell crawling and dendrite probing is visible.
Supplementary Video 4
Tolerizing interactions 1-6 h after T cell transfer (MOV 2598 kb)
Antigen-specific T cells (orange) move more slowly than non-specific T cells (red), and are more frequently arrested on dendritic cells (green). Tracks of specific EGFP-OT-II cells are cyan, and tracks of nonspecific ECFP-T cells are purple.
Supplementary Video 5
Arrest of antigen-specific T cells near HEVs (MOV 2119 kb)
Antigen-specific EGFP-T cells (cyan) found arrested on the basal surface HEVs. Non-specific T cells (blue) move normally. Tracks of EGFP-OT-II cells are green, tracks of ECFP-T cells are blue, and DCs are green. HEVs (red) were visualized by i.v. injection of Alexa 594-labeled MECA-79 antibody, which targets peripheral node addressin (PNAd).
Supplementary Video 6
Tolerizing interactions 6-12 h after T cell transfer (MOV 1722 kb)
Antigen-specific T cells (orange) move more slowly than non-specific T cells (red), and are more frequently arrested on dendritic cells (green). Tracks of specific EGFP-OT-II cells are cyan, and tracks of nonspecific ECFP-T cells are purple. Antigen-specific T cells are less arrested than at 1-6 h.
Supplementary Video 7
Tolerizing interactions 12-18 h after T cell transfer. (MOV 2894 kb)
Antigen-specific T cells (orange) and non-specific T cells (red) interact with DCs (green) as they move throughout the T cell area. Tracks of specific EGFP-OT-II cells are cyan, and tracks of nonspecific ECFP-T cells are purple. Antigen-specific T cells have largely recovered in speed, but still move slightly slower than nonspecific T cells.
Supplementary Video 8
Priming interactions 1-6 h after T cell transfer. (MOV 1851 kb)
Antigen-specific T cells (orange) move more slowly than nonspecific T cells (red) and are more frequently arrested on DCs (green). Tracks of specific EGFP-OT-II cells are cyan, and tracks of nonspecific ECFP-T cells are purple.
Supplementary Video 9
Priming interactions 6-12 h after T cell transfer. (MOV 2408 kb)
Antigen-specific T cells (orange) move more slowly than nonspecific T cells (red) and are more frequently arrested on DCs (green). Tracks of specific EGFP-OT-II cells are cyan, and tracks of nonspecific ECFP-T cells are purple. EGFP-OT-II cells are equally arrested at 6-12 h as they are at 1-6 h after transfer.
Supplementary Video 10
Priming interactions 12-18 h after T cell transfer. (MOV 2606 kb)
Antigen-specific T cells (orange) move more slowly than nonspecific T cells (red) and are more frequently arrested on DCs (green). Tracks of specific EGFP-OT-II cells are cyan, and tracks of nonspecific ECFP-T cells are purple. EGFP-OT-II cells have partially recovered in speed, but are still more arrested than the nonspecific ECFP-T cells.
Supplementary Video 11
T cell cluster formation (MOV 3205 kb)
This phenomenon is relatively rare with antigen targeted to DEC-205, as compared to cell-bound antigen delivered by a small fraction of APCs in the lymph node. The cluster seen here is composed of antigen-specific EGFP-OT-II T cells (orange) and not wild-type ECFP T cells (red).
Supplementary Video 12
T cell arrest on fluorescent and non-fluorescent dendritic cells (MOV 1556 kb)
T cell arrest on EYFP- cells was often observed. This may reflect DCs that express relatively low levels of EYFP or processes of EYFP+ DCs too thin to be visualized by multiphoton microscopy. The rightmost T cell is seen contacting an EYFP+ DC, then detaching and arresting nearby, possibly on the surface of a non-fluorescent APC.
Rights and permissions
About this article
Cite this article
Shakhar, G., Lindquist, R., Skokos, D. et al. Stable T cell–dendritic cell interactions precede the development of both tolerance and immunity in vivo. Nat Immunol 6, 707–714 (2005). https://doi.org/10.1038/ni1210
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1210
This article is cited by
-
Rapid video-based deep learning of cognate versus non-cognate T cell-dendritic cell interactions
Scientific Reports (2022)
-
High-resolution imaging of living gut mucosa: lymphocyte clusters beneath intestinal M cells are highly dynamic structures
Cell and Tissue Research (2020)
-
Dendritic cells as gatekeepers of tolerance
Seminars in Immunopathology (2017)
-
The bullseye synapse formed between CD4+ T‐cell and staphylococcal enterotoxin B‐pulsed dendritic cell is a suppressive synapse in T‐cell response
Immunology & Cell Biology (2015)
-
The atypical chemokine receptor CCRL1 shapes functional CCL21 gradients in lymph nodes
Nature Immunology (2014)