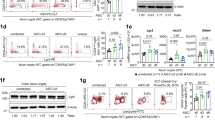
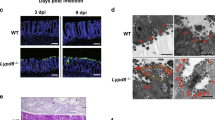
Abstract
Epithelial tissues continually undergo apoptosis. Commensal organisms that inhabit the epithelium influence tissue homeostasis, in which regulatory T cells (Treg cells) have a central role. However, the physiological importance of epithelial cell apoptosis and how the number of Treg cells is regulated are both incompletely understood. Here we found that apoptotic epithelial cells negatively regulated the commensal-stimulated proliferation of Treg cells. Gut commensals stimulated CX3CR1+CD103−CD11b+ dendritic cells (DCs) to produce interferon-β (IFN-β), which augmented the proliferation of Treg cells in the intestine. Conversely, phosphatidylserine exposed on apoptotic epithelial cells suppressed IFN-β production by the DCs via inhibitory signaling mediated by the cell-surface glycoprotein CD300a and thus suppressed Treg cell proliferation. Our findings reveal a regulatory role for apoptotic epithelial cells in maintaining the number of Treg cell and tissue homeostasis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Accession codes
References
Belkaid, Y. & Hand, T.W. Role of the microbiota in immunity and inflammation. Cell 157, 121–141 (2014).
Bauer, H., Horowitz, R.E., Levenson, S.M. & Popper, H. The response of the lymphatic tissue to the microbial flora. Studies on germfree mice. Am. J. Pathol. 42, 471–483 (1963).
Kamada, N., Seo, S.U., Chen, G.Y. & Nunez, G. Role of the gut microbiota in immunity and inflammatory disease. Nat. Rev. Immunol. 13, 321–335 (2013).
Hooper, L.V., Littman, D.R. & Macpherson, A.J. Interactions between the microbiota and the immune system. Science 336, 1268–1273 (2012).
Atarashi, K. et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 331, 337–341 (2011).
Atarashi, K. et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 500, 232–236 (2013).
Mazmanian, S.K., Round, J.L. & Kasper, D.L. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature 453, 620–625 (2008).
Furusawa, Y. et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504, 446–450 (2013).
Arpaia, N. et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504, 451–455 (2013).
Singh, N. et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 40, 128–139 (2014).
Ravichandran, K.S. & Lorenz, U. Engulfment of apoptotic cells: signals for a good meal. Nat. Rev. Immunol. 7, 964–974 (2007).
Nagata, S., Hanayama, R. & Kawane, K. Autoimmunity and the clearance of dead cells. Cell 140, 619–630 (2010).
Zhou, Z. New phosphatidylserine receptors: clearance of apoptotic cells and more. Dev. Cell 13, 759–760 (2007).
Vincent, J.P., Fletcher, A.G. & Baena-Lopez, L.A. Mechanisms and mechanics of cell competition in epithelia. Nat. Rev. Mol. Cell Biol. 14, 581–591 (2013).
Eisenhoffer, G.T. et al. Crowding induces live cell extrusion to maintain homeostatic cell numbers in epithelia. Nature 484, 546–549 (2012).
Borrego, F. The CD300 molecules: an emerging family of regulators of the immune system. Blood 121, 1951–1960 (2013).
Yotsumoto, K. et al. Paired activating and inhibitory immunoglobulin-like receptors, MAIR-I and MAIR-II, regulate mast cell and macrophage activation. J. Exp. Med. 198, 223–233 (2003).
Okoshi, Y. et al. Requirement of the tyrosines at residues 258 and 270 of MAIR-I in inhibitory effect on degranulation from basophilic leukemia RBL-2H3. Int. Immunol. 17, 65–72 (2005).
Simhadri, V.R. et al. Human CD300a binds to phosphatidylethanolamine and phosphatidylserine, and modulates the phagocytosis of dead cells. Blood 119, 2799–2809 (2012).
Nakahashi-Oda, C., Tahara-Hanaoka, S., Honda, S.I., Shibuya, K. & Shibuya, A. Identification of phosphatidylserine as a ligand for the CD300a immunoreceptor. Biochem. Biophys. Res. Commun. 417, 646–650 (2011).
Nakahashi-Oda, C. et al. Apoptotic cells suppress mast cell inflammatory responses via the CD300a immunoreceptor. J. Exp. Med. 209, 1493–1503 (2012).
Hanayama, R. et al. Identification of a factor that links apoptotic cells to phagocytes. Nature 417, 182–187 (2002).
Littman, D.R. & Rudensky, A.Y. Th17 and regulatory T cells in mediating and restraining inflammation. Cell 140, 845–858 (2010).
Geuking, M.B. et al. Intestinal bacterial colonization induces mutualistic regulatory T cell responses. Immunity 34, 794–806 (2011).
Zanoni, I. et al. CD14 controls the LPS-induced endocytosis of Toll-like receptor 4. Cell 147, 868–880 (2011).
Coombes, J.L. et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J. Exp. Med. 204, 1757–1764 (2007).
Niess, J.H. et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science 307, 254–258 (2005).
Vallon-Eberhard, A., Landsman, L., Yogev, N., Verrier, B. & Jung, S. Transepithelial pathogen uptake into the small intestinal lamina propria. J. Immunol. 176, 2465–2469 (2006).
Kawai, T. & Akira, S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 11, 373–384 (2010).
Trinchieri, G. Type I interferon: friend or foe? J. Exp. Med. 207, 2053–2063 (2010).
An, H. et al. SHP-2 phosphatase negatively regulates the TRIF adaptor protein-dependent type I interferon and proinflammatory cytokine production. Immunity 25, 919–928 (2006).
Sly, L.M. et al. SHIP prevents lipopolysaccharide from triggering an antiviral response in mice. Blood 113, 2945–2954 (2009).
Sly, L.M., Rauh, M.J., Kalesnikoff, J., Song, C.H. & Krystal, G. LPS-induced upregulation of SHIP is essential for endotoxin tolerance. Immunity 21, 227–239 (2004).
González-Navajas, J.M., Lee, J., David, M. & Raz, E. Immunomodulatory functions of type I interferons. Nat. Rev. Immunol. 12, 125–135 (2012).
Katakura, K. et al. Toll-like receptor 9-induced type I IFN protects mice from experimental colitis. J. Clin. Invest. 115, 695–702 (2005).
Kawashima, T. et al. Double-stranded RNA of intestinal commensal but not pathogenic bacteria triggers production of protective interferon-β. Immunity 38, 1187–1197 (2013).
Monteleone, G., Pallone, F. & MacDonald, T.T. Emerging immunological targets in inflammatory bowel disease. Curr. Opin. Pharmacol. 11, 640–645 (2011).
Mannon, P.J. et al. Suppression of inflammation in ulcerative colitis by interferon-β-1a is accompanied by inhibition of IL-13 production. Gut. 60, 449–455 (2011).
Kole, A. et al. Type I IFNs regulate effector and regulatory T cell accumulation and anti-inflammatory cytokine production during T cell-mediated colitis. J. Immunol. 191, 2771–2779 (2013).
Lee, S.E. et al. Type I interferons maintain Foxp3 expression and T-regulatory cell functions under inflammatory conditions in mice. Gastroenterology 143, 145–154 (2012).
Srivastava, S., Koch, M.A., Pepper, M. & Campbell, D.J. Type I interferons directly inhibit regulatory T cells to allow optimal antiviral T cell responses during acute LCMV infection. J. Exp. Med. 211, 961–974 (2014).
Wang, Y. et al. Th2 lymphoproliferative disorder of LatY136F mutant mice unfolds independently of TCR-MHC engagement and is insensitive to the action of Foxp3+ regulatory T cells. J. Immunol. 180, 1565–1575 (2008).
Rodríguez, C.I. et al. High-efficiency deleter mice show that FLPe is an alternative to Cre-loxP. Nat. Genet. 25, 139–140 (2000).
Trowbridge, I.S., Lesley, J. & Schulte, R. Murine cell surface transferrin receptor: studies with an anti-receptor monoclonal antibody. J. Cell. Physiol. 112, 403–410 (1982).
Graves, C.L. et al. A method for high purity intestinal epithelial cell culture from adult human and murine tissues for the investigation of innate immune function. J. Immunol. Methods 414, 20–31 (2014).
Aldini, R. et al. Antiinflammatory effect of phytosterols in experimental murine colitis model: prevention, induction, remission study. PLoS ONE 9, e108112 (2014).
Spergel, J.M., Mizoguchi, E., Oettgen, H., Bhan, A.K. & Geha, R.S. Roles of TH1 and TH2 cytokines in a murine model of allergic dermatitis. J. Clin. Invest. 103, 1103–1111 (1999).
Nigo, Y.I. et al. Regulation of allergic airway inflammation through Toll-like receptor 4-mediated modification of mast cell function. Proc. Natl. Acad. Sci. USA 103, 2286–2291 (2006).
Acknowledgements
We thank B. Malissen (UM2 Aix-Marseille Université) for Foxp3eGFP mice; E. Nakayama (Okayama University) for anti-CD25; M. Tanaka (Research Center for Allergy and Immunology, Yokohama, Japan) for MFG-E8(D89E) and MFG-E8(EPT); K. Honda and G. Nunez for discussions; S. Tochihara and Y. Nomura for secretarial assistance; and F. Abe and R. Hirochika for technical assistance. Supported by Japan Society for the Promotion of Science (KAKENHI), Core Research for Evolutional Science and Technology, Japan Agency for Medical Research and Development–Core Research for Evolutional Science and Technology and Uehara Memorial Foundation (A.S.).
Author information
Authors and Affiliations
Contributions
C.N.-O. conducted the experiments, analyzed the data, and wrote the paper; K.G.S.U. performed immunohistochemical studies and analyzed dermatitis; Yo.N. performed in vivo studies of DSS-induced colitis; Yu.N. analyzed signal transduction via TLR4 and CD300a; N.T., H.M. and S.I. performed the experiments of S. typhimurium infection, allergic airway inflammation and DSS-induced colitis, respectively; S.T.-H., S.H. and K.S. analyzed the data; and A.S. supervised the overall project and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Treg cell populations are comparable in wild-type and Cd300a−/− mice in organs that lack a resident microbiota.
Flow cytometry of Foxp3 expression in CD4+ T cells of the small intestine (a) and spleen and mesenteric and axillar lymph node (b) from Cd300 a−/− or wild-type (WT) mice raised under specific-pathogen-free (SPF) conditions. Error bars indicate SEM. *, P < 0.05. NS, not significant.
Supplementary Figure 2 Immunohistochemical staining of intestine, skin and lungs.
Flag-tagged D89E MFG-E8 or EPT MFG-E8 was infused rectally (colon) into, applied topically (skin) on, and injected intranasally (lung) into mice; fixed sections were stained by fluorescein isothiocyanate (FITC)-conjugated anti-FLAG mAb and DAPI, and then analyzed by fluorescence microscopy. White dashed lines indicate the surface of epithelium. White bars indicate scale (20μm). Data are representative of more than three experiments.
Supplementary Figure 3 CD300a+ cells interact with epithelial apoptotic cells.
(a) RAW267.4 transfectants stably expressing CD300a or MAIR-II directly fused with DS-Red (red) at the C-terminus were cocultured with apoptotic thymocytes that had been stained with PSVue 480 (green), and analysed by immunofluorescence microscopy. (b-e) Mice were infused rectally or applied to the dorsum or administered intranasally with Flag-tagged D89E MFG-E8 (b, e) or PSVue 480 (d). Tissue sections from the colon, skin and lung of these mice (b-d) or CD11c-GFPmice (c) were stained with Alexa 647-conjugated anti-E-cadherin mAb (b), Alexa 546-conjugated anti-CD300a mAb (c-e), FITC-conjugated anti-CD207 mAb (c), Alexa 488-conjugated anti-GFP Ab (c), and/or FITC-conjugated anti-Flag mAb (b, e), followed by the staining with DAPI (b-e) and then analyzed by fluorescence microscopy. White arrows indicate CD300a-expressing DC or LC (c) and possible interactions between CD300a-expressing cells and epithelial apoptotic cells (d, e). White bars indicate scale (10 μm). Data are representative of three (a) and five (b-e) experiments. (f) Scheme of in vivo imaging analysis using probe-based confocal laser endomicroscopy of colon.
Supplementary Figure 4 Characterization of CD300a+ cells.
(a, b) Cells of the colonic lamina propria from WT (a and b) and CD300a−/− (a) mice were stained with Alexa 700-conjugated anti-CD45.2, propidium iodide (PI), Horizon V500-conjugated MHC class II, APC-Cy7-conjugated anti-CD11b, PE-Cy7-conjugated anti-CD11c, FITC-conjugated anti-CD103, PE-conjugated F4/80, and either APC-conjugated anti-CD300a (a), FITC and PE-conjugated mAbs indicated (b), or control Ab (a, b) and analyzed by means of flow cytometry. Data are representative of three mice. (c) Cells obtained from the colonic lamina propria, peritoneal cavity (PEC) or spleen of WT, CD300a−/−, Cd300afl/fl and Cd300afl/flItgax-Cre mice were stained as described above or with PE-conjugated anti-c-Kit, FITC-conjugated anti-FcεRI and PE-conjugated anti-F4/80. CD300a expressions were analyzed by means of flow cytometry. (d) Microarray analysis performed on the mRNA of CD11b+ DCs sorted from the colonic lamina propria of WT and Cd300a−/− of germ-free (GF) and SPF mice (pooled from 4 mice each). The heat map demonstrates changes in the expression levels of the indicated genes.
Supplementary Figure 5 Cd300a−/− mice show attenuated DSS-induced colitis and allergic inflammation in the skin and lungs.
Rag1−/− (n = 10) and Rag1−/−Cd300a−/−mice (n = 8) were treated with 2.5% DSS and monitored for loss of body weight. Error bars, SEM. Data are representative of two independent experiments. (b-e) WT and Cd300a−/− mice were treated with 2.5% DSS for 7 days. Cells were isolated from the lamina propria before and during DSS-treatment and stained with anti-CD45.2, anti-CD4, and either PE-conjugated anti-Foxp3 (b), FITC-conjugated anti-IFN-γ, PE-conjugated anti-IL-17 or APC-conjugated anti-IL-4 mAb (c). The proportions of Foxp3+ cells in CD4+ T cells (b) and CD4+ T cells producing IFN-γ, IL-17, IL-4 or IL-10 were calculated (c). (d) Cells were isolated from the lamina propria beforeand 7 days after the start of DSS treatment and cultured overnight. Cytokine levels were determined by ELISA in the culture supernatants. (e) CD11b+ DCs were isolated from the colonia lamina propria cells by flow cytometry 7 days after DSS treatment and examined for the expressions of cytokine mRNAs by quantitative RT-PCR. (f) Mice were injected intravenously with 500 μg of control or anti-CD25 mAb on day 0 and treated orally with 2.5% DSS for 7 days. On day 7, Foxp3 expression in the CD4+ T cells of the colonic lamina propria was analyzed by means of flow cytometry. (g-j) WT and Cd300a−/− mice were treated topically with LPS and ovalbumin (OVA) on the dorsal skin to induce allergic inflammation (g). On day 14, skin samples were dissected, analyzed histologically after hematoxylin and eosin staining (b and d), and evaluated for epidermal thickness (h). Serum IgE levels were measured with an ELISA (i). (j) WT and Cd300a−/− mice were injected intraperitoneally with anti-CD25 mAb or control mAb to deplete Treg cells on days -4, 3, and 10. (k-n) WT and Cd300a−/− mice were intranasally treated with LPS and OVA on the indicated days (k), and lungs were analyzed histologically after staining with hematoxylin and eosin or periodic acid–Schiff on day 18 (l). The number of Siglec F+ eosinophils in the lung tissues was determined by means of flow cytometry (m), and serum IgE levels were analyzed with an ELISA (n). Error bars, SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001. NS, not significant.
Supplementary Figure 6 The increase in the size of the Treg cell population in the skin and lungs of Cd300a−/− mice is dependent on TRIF.
Cells isolated from the skin and lung of Cd300a−/−, Ticam1−/− and Ticam1−/−Cd300a−/− mice were analyzed for Foxp3+ expression in CD4+ T cells by means of flow cytometry, as described in the legend to Figure 1. Error bars, SEM. NS, not significant. Data are representative of two independent experiments.
Supplementary Figure 7 Involvement of CD300a in the suppression of IFN-b production by BMDCs.
(a) Bone marrow-derived cultured DCs (BMDCs) induced by GM-CSF and IL4 for 7 d were stained with PE-conjugated anti-CD11c, PECy7-conjugated anti-MHC classII, FITC-conjugated anti-CD11b, and Alexa647-conjugated anti-CD300a. CD11c+MHC class II+ cells were analyzed for expression of CD11b and CD300a by means of flow cytometry. (b) The percentage of apoptotic cells in cultures of BMDCs was analyzed by means of flow cytometry. Phosphatidylserine (PS) was stained by using a FITC-conjugated anti-PS antibody. Data are representative of 3 mice. (c) BMDCs induced from bone marrow cells of WT, Tlr3−/−, and Tlr4−/− mice were stimulated with fecal contents and treated with a neutralizing anti-CD300a mAb. Subsequent mRNA expression of IFN-b was measured by means of quantitative PCR analysis. Error bars, SEM. *, P < 0.05; **, P < 0.01. NS, not significant. ND, not detected. Data are representative of two independent experiments.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 and Supplementary Table 1 (PDF 6276 kb)
Rights and permissions
About this article
Cite this article
Nakahashi-Oda, C., Udayanga, K., Nakamura, Y. et al. Apoptotic epithelial cells control the abundance of Treg cells at barrier surfaces. Nat Immunol 17, 441–450 (2016). https://doi.org/10.1038/ni.3345
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.3345