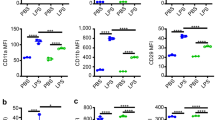
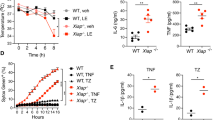
Abstract
Transcription factor NF-κB and its activating kinase IKKβ are associated with inflammation and are believed to be critical for innate immunity. Despite the likelihood of immune suppression, pharmacological blockade of IKKβ–NF-κB has been considered as a therapeutic strategy. However, we found neutrophilia in mice with inducible deletion of IKKβ (IkkβΔ mice). These mice had hyperproliferative granulocyte-macrophage progenitors and pregranulocytes and a prolonged lifespan of mature neutrophils that correlated with the induction of genes encoding prosurvival molecules. Deletion of interleukin 1 receptor 1 (IL-1R1) in IkkβΔ mice normalized blood cellularity and prevented neutrophil-driven inflammation. However, IkkβΔIl1r1−/− mice, unlike IkkβΔ mice, were highly susceptible to bacterial infection, which indicated that signaling via IKKβ–NF-κB or IL-1R1 can maintain antimicrobial defenses in each other's absence, whereas inactivation of both pathways severely compromises innate immunity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Segal, A.W. How neutrophils kill microbes. Annu. Rev. Immunol. 23, 197–223 (2005).
Klebanoff, S.J. & Clark, R.A. The Neutrophil: Function and Clinical Disorders 5–162 (Elsevier/North-Holland, Amsterdam, 1978).
Quie, P.G. The phagocytic system in host defense. Scand. J. Infect. Dis. Suppl 24, 30–32 (1980).
Burg, N.D. & Pillinger, M.H. The neutrophil: function and regulation in innate and humoral immunity. Clin. Immunol. 99, 7–17 (2001).
Brinkmann, V. et al. Neutrophil extracellular traps kill bacteria. Science 303, 1532–1535 (2004).
von Kockritz-Blickwede, M. & Nizet, V. Innate immunity turned inside-out: antimicrobial defense by phagocyte extracellular traps. J. Mol. Med. 87, 775–783 (2009).
Lundqvist-Gustafsson, H. & Bengtsson, T. Activation of the granule pool of the NADPH oxidase accelerates apoptosis in human neutrophils. J. Leukoc. Biol. 65, 196–204 (1999).
Nathan, C. Neutrophils and immunity: challenges and opportunities. Nat. Rev. Immunol. 6, 173–182 (2006).
Metcalf, D. Stem cells, pre-progenitor cells and lineage-committed cells: are our dogmas correct? Ann. NY Acad. Sci. 872, 289–303 (1999).
Akashi, K., Traver, D., Miyamoto, T. & Weissman, I.L. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature 404, 193–197 (2000).
Yang, L. et al. Identification of Lin−Sca1+kit+CD34+Flt3− short-term hematopoietic stem cells capable of rapidly reconstituting and rescuing myeloablated transplant recipients. Blood 105, 2717–2723 (2005).
Metcalf, D. & Nicola, N.A. The Hemopoietic Colony Stimulating Factors 109–165 (Cambridge University Press, Cambridge, UK, 1995).
Barnes, P.J. & Karin, M. Nuclear factor-κB: a pivotal transcription factor in chronic inflammatory diseases. N. Engl. J. Med. 336, 1066–1071 (1997).
Greten, F.R. & Karin, M. The IKK/NF-κB activation pathway-a target for prevention and treatment of cancer. Cancer Lett. 206, 193–199 (2004).
Akira, S., Uematsu, S. & Takeuchi, O. Pathogen recognition and innate immunity. Cell 124, 783–801 (2006).
Bonizzi, G. & Karin, M. The two NF-κB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 25, 280–288 (2004).
Hacker, H. & Karin, M. Regulation and function of IKK and IKK-related kinases. Sci. STKE 2006, re13 (2006).
Straus, D.S. Design of small molecules targeting transcriptional activation by NF-κB: overview of recent advances. Expert Opin. Drug Discov. 4, 823–836 (2009).
Baud, V. & Karin, M. Is NF-κB a good target for cancer therapy? Hopes and pitfalls. Nat. Rev. Drug Discov. 8, 33–40 (2009).
Nagashima, K. et al. Rapid TNFR1-dependent lymphocyte depletion in vivo with a selective chemical inhibitor of IKKβ. Blood 107, 4266–4273 (2006).
Greten, F.R. et al. NF-κB is a negative regulator of IL-1β secretion as revealed by genetic and pharmacological inhibition of IKKβ. Cell 130, 918–931 (2007).
Mbalaviele, G. et al. A novel, highly selective, tight binding IκB kinase-2 (IKK-2) inhibitor: a tool to correlate IKK-2 activity to the fate and functions of the components of the nuclear factor-κB pathway in arthritis-relevant cells and animal models. J. Pharmacol. Exp. Ther. 329, 14–25 (2009).
Kuhn, R., Schwenk, F., Aguet, M. & Rajewsky, K. Inducible gene targeting in mice. Science 269, 1427–1429 (1995).
Ruocco, M.G. et al. IκB kinase (IKK)β, but not IKKα, is a critical mediator of osteoclast survival and is required for inflammation-induced bone loss. J. Exp. Med. 201, 1677–1687 (2005).
Hestdal, K. et al. Characterization and regulation of RB6–8C5 antigen expression on murine bone marrow cells. J. Immunol. 147, 22–28 (1991).
Dinarello, C.A. Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 27, 519–550 (2009).
Vitale, C. et al. Engagement of p75/AIRM1 or CD33 inhibits the proliferation of normal or leukemic myeloid cells. Proc. Natl. Acad. Sci. USA 96, 15091–15096 (1999).
Chen, C., Edelstein, L.C. & Gelinas, C. The Rel/NF-κB family directly activates expression of the apoptosis inhibitor Bcl-xL . Mol. Cell. Biol. 20, 2687–2695 (2000).
Boise, L.H. et al. Bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell 74, 597–608 (1993).
Fu, L. et al. Constitutive NF-κB and NFAT activation leads to stimulation of the BLyS survival pathway in aggressive B-cell lymphomas. Blood 107, 4540–4548 (2006).
Resnitzky, D., Hengst, L. & Reed, S.I. Cyclin A-associated kinase activity is rate limiting for entrance into S phase and is negatively regulated in G1 by p27Kip1. Mol. Cell. Biol. 15, 4347–4352 (1995).
Catlett-Falcone, R. et al. Constitutive activation of Stat3 signaling confers resistance to apoptosis in human U266 myeloma cells. Immunity 10, 105–115 (1999).
He, G. et al. Hepatocyte IKKβ/NF-κB inhibits tumor promotion and progression by preventing oxidative stress-driven STAT3 activation. Cancer Cell 17, 286–297 (2010).
Horwitz, B.H., Scott, M.L., Cherry, S.R., Bronson, R.T. & Baltimore, D. Failure of lymphopoiesis after adoptive transfer of NF-κB-deficient fetal liver cells. Immunity 6, 765–772 (1997).
Grossmann, M. et al. The combined absence of the transcription factors Rel and RelA leads to multiple hemopoietic cell defects. Proc. Natl. Acad. Sci. USA 96, 11848–11853 (1999).
Senftleben, U., Li, Z.W., Baud, V. & Karin, M. IKKβ is essential for protecting T cells from TNFα-induced apoptosis. Immunity 14, 217–230 (2001).
Stanley, E.R., Bartocci, A., Patinkin, D., Rosendaal, M. & Bradley, T.R. Regulation of very primitive, multipotent, hemopoietic cells by hemopoietin-1. Cell 45, 667–674 (1986).
Ueda, Y., Cain, D.W., Kuraoka, M., Kondo, M. & Kelsoe, G. IL-1R type I-dependent hemopoietic stem cell proliferation is necessary for inflammatory granulopoiesis and reactive neutrophilia. J. Immunol. 182, 6477–6484 (2009).
Greten, F.R. et al. IKKβ links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 118, 285–296 (2004).
Lane, A.A. & Ley, T.J. Neutrophil elastase is important for PML-retinoic acid receptor α activities in early myeloid cells. Mol. Cell. Biol. 25, 23–33 (2005).
Jamieson, C.H. et al. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N. Engl. J. Med. 351, 657–667 (2004).
Genovese, M.C. et al. Combination therapy with etanercept and anakinra in the treatment of patients with rheumatoid arthritis who have been treated unsuccessfully with methotrexate. Arthritis Rheum. 50, 1412–1419 (2004).
Kuida, K. et al. Altered cytokine export and apoptosis in mice deficient in interleukin-1 β converting enzyme. Science 267, 2000–2003 (1995).
Hsu, L.C. et al. A NOD2-NALP1 complex mediates caspase-1-dependent IL-1β secretion in response to Bacillus anthracis infection and muramyl dipeptide. Proc. Natl. Acad. Sci. USA 105, 7803–7808 (2008).
Temkin, V., Huang, Q., Liu, H., Osada, H. & Pope, R.M. Inhibition of ADP/ATP exchange in receptor-interacting protein-mediated necrosis. Mol. Cell. Biol. 26, 2215–2225 (2006).
Kranich, J. et al. Follicular dendritic cells control engulfment of apoptotic bodies by secreting Mfge8. J. Exp. Med. 205, 1293–1302 (2008).
Abrahamsson, A.E. et al. Glycogen synthase kinase 3β missplicing contributes to leukemia stem cell generation. Proc. Natl. Acad. Sci. USA 106, 3925–3929 (2009).
Breckpot, K. et al. Lentivirally transduced dendritic cells as a tool for cancer immunotherapy. J. Gene Med. 5, 654–667 (2003).
Acknowledgements
We thank K. Kaushansky, G. Wulf and N. Zatula for advice and support; L. Jerabek and A. Mosley for laboratory and animal management; C. Richter and N. Teja for antibody production; and the National Clinical Trial & Research Center at National Taiwan University Hospital, and the Bioinformatics and Biostatistics Core, Research Center for Medical Excellence at National Taiwan University, Taiwan, for microarray services and assistance in data analysis. Supported by the US National Institutes of Health (AI043477 to M.K., AI77780 to V.N. and U01HL099999-02 to I.L.W.), National Taiwan University (L.-C.H.), National Health Research Institutes, Taiwan (NHRI-EX99-9825SC to L.-C.H.)., Leukemia and Lymphoma Society of America (T.E.), California Institute for Regenerative Medicine (J.S.) and American Cancer Society (M.K. and I.L.W.).
Author information
Authors and Affiliations
Contributions
J.S. and A.M.T. contributed equally to this work. L.-C.H. and T.E. designed and did most of the experiments; M.K. helped in designing experiments; M.K., T.E. and L.-C.H. wrote the paper; J.S. and I.L.W. planned and did most of the progenitor cell analyses; A.M.T. and V.N. planned and did the bacterial killing experiments; and C.-Y.L., T.-Y.L., G.-Y.Y., L.-C.L., V.T., U.S. and T.A. helped with some of the experiments.
Corresponding authors
Ethics declarations
Competing interests
M.K. holds several US and international patents on the development and use of IKKβ inhibitors; I.L.W. has stock in Amgen, is a cofounder of Cellerant and Stem Cells and is also a consultant for Stem Cells.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10, Supplementary Table 1 and Supplementary Methods (PDF 2682 kb)
Rights and permissions
About this article
Cite this article
Hsu, LC., Enzler, T., Seita, J. et al. IL-1β-driven neutrophilia preserves antibacterial defense in the absence of the kinase IKKβ. Nat Immunol 12, 144–150 (2011). https://doi.org/10.1038/ni.1976
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.1976
This article is cited by
-
Divergent functions of NLRP3 inflammasomes in cancer: a review
Cell Communication and Signaling (2023)
-
The NF-κB Pathway: a Focus on Inflammatory Responses in Spinal Cord Injury
Molecular Neurobiology (2023)
-
Deletion of IKK2 in haematopoietic cells of adult mice leads to elevated interleukin-6, neutrophilia and fatal gastrointestinal inflammation
Cell Death & Disease (2021)
-
Serum amyloid P component is an essential element of resistance against Aspergillus fumigatus
Nature Communications (2021)
-
NF-κB-p62-NRF2 survival signaling is associated with high ROR1 expression in chronic lymphocytic leukemia
Cell Death & Differentiation (2020)