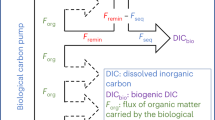
Abstract
A fraction of the carbon captured by phytoplankton in the sunlit surface ocean sinks to depth as dead organic matter and faecal material. The microbial breakdown of this material in the subsurface ocean generates carbon dioxide. Collectively, this microbially mediated flux of carbon from the atmosphere to the ocean interior is termed the biological pump. In recent decades it has become clear that the composition of the phytoplankton community in the surface ocean largely determines the quantity and quality of organic matter that sinks to depth. This settling organic matter, however, is not sufficient to meet the energy demands of microbes in the dark ocean. Two additional sources of organic matter have been identified: non-sinking organic particles of debated origin that escape capture by sediment traps and exhibit stable concentrations throughout the dark ocean, and microbes that convert inorganic carbon into organic matter. Whether these two sources can together account for the significant mismatch between organic matter consumption and supply in the dark ocean remains to be seen. It is clear, however, that the microbial community of the deep ocean works in a fundamentally different way from surface water communities.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Ducklow, H. W., Steinberg, D. K. & Buesseler, K. O. Upper ocean carbon export and the biological pump. Oceanography 14, 50–58 (2001).
Armstrong, R. A., Peterson, M. L., Lee, C. & Wakeham, S. G. Settling velocity sprectra and the ballast ratio hypothesis. Deep Sea Res. II 56, 1470–1478 (2009).
Steinberg, D. K. et al. Bacterial vs. zooplankton control of sinking particle flux in the ocean's twilight zone. Limnol. Oceanogr. 53, 1327–1338 (2008).
Reinthaler, T. et al. Prokaryotic respiration and production in the meso- and bathypelagic realm of the eastern and western North Atlantic basin. Limnol. Oceanogr. 51, 1262–1273 (2006).
Alldredge, A. L. & Jackson, G. A. Aggregation in marine systems. Deep Sea Res. II 42, 1–7 (1995).
Obernosterer, I. & Herndl, G. J. Phytoplankton extracellular release and bacterial growth: dependence on the inorganic N:P ratio. Mar. Ecol. Prog. Ser. 116, 247–257 (1995).
Verdugo, P. et al. The oceanic gel phase: a bridge in the DOM-POM continuum. Mar. Chem. 92, 67–85 (2004).
Alldredge, A., Granata, T. C., Gotschalk, C. C. & Dickey, T. D. The physical strength of marine snow and its implications for particle dissagregation in the ocean. Limnol. Oceanogr. 35, 1415–1428 (1990).
Stoderegger, K. & Herndl, G. J. Production and release of bacterial capsular material and its subsequent utilization by marine bacterioplankton. Limnol. Oceanogr. 43, 877–884 (1998).
Stoderegger, K. E. & Herndl, G. J. Production of exopolymer particles by marine bacterioplankton under contrasting turbulence conditions. Mar. Ecol. Prog. Ser. 189, 9–16 (1999).
Martin, J. H., Knauer, G. A., Karl, D. M. & Broenkow, W. W. VERTEX: carbon cycling in the northeast Pacific. Deep-Sea Res. 34, 267–285 (1987).
Kwon, E. Y., Primeau, F. & Sarmiento, J. L. The impact of remineralization depth on the air-sea carbon balance. Nature Geosci. 2, 630–635 (2009).
Buesseler, K. O. & Boyd, P. W. Shedding light on processes that control particle export and flux attenuation in the twilight zone of the open ocean. Limnol. Oceanogr. 54, 1210–1232 (2009).
Smetacek, V. The giant diatom dump. Nature 406, 574–575 (2000).
Shatova, O., Koweek, D., Conte, M. H. & Weber, J. C. Contribution of zooplankton fecal pellets to deep ocean particle flux in the Sargasso Sea assessed using quantitative image analysis. J. Plankton Res. 34, 905–921 (2012).
Richardson, T. L. & Jackson, G. A. Small phytoplankton and carbon export from the surface ocean. Science 315, 838–840 (2007).
Guidi, L. et al. Effects of phytoplankton community on production, size and export of large aggregates: A world-ocean analysis. Limnol. Oceanogr. 54, 1951 (2009).
Bochdansky, A. B., Aken, H. M. v. & Herndl, G. J. Role of macroscopic particles in deep-sea oxygen consumption. Proc. Natl Acad. Sci. USA 107, 8287–8291 (2010).
Lampitt, R. S. & Antia, A. N. Particle flux in deep sea: regional characteristics and temporal variability. Deep Sea Res. I 44, 1377–1403 (1997).
Buesseler, K. O. Do upper-ocean sediment traps provide an accurate record of particle flux? Nature 353, 420–423 (1991).
Honjo, S. in Particle Flux in the Ocean. SCOPE Vol. 57 (eds Ittekot, V., Schäfer, P., Honjo, S. & Depetris, P. J.) 91–254 (Wiley, 1996).
Honjo, S. & Manganini, S. J. Annual biogenic particle fluxes to the interior of the North Atlantic Ocean; studied at 34° N 21° W and 48°N 21° W. Deep Sea Res. 40, 587–607 (1993).
Buesseler, K. O. et al. A comparison of the quantity and composition of material caught in a neutrally buoyant versus surface-tethered sediment trap. Deep Sea Res. I 47, 277–294 (2000).
Graham, G. W. & Smith, W. A. M. N. The application of holography to the analysis of size and settling velocity of suspended cohesive sediments. Limnol. Oceanogr. Methods 8 (1–15) (2010).
Baltar, F. et al. Significance of non-sinking particulate organic carbon and dark CO2 fixation to heterotrophic carbon demand in the mesopelagic northeast Atlantic. Geophys. Res. Lett. 37, L09602 (2010).
Baltar, F., Aristegui, J., Gasol, J. M., Sintes, E. & Herndl, G. J. Evidence of prokaryotic metabolism on suspended particulate organic matter in the dark waters of the subtropical North Atlantic. Limnol. Oceanogr. 54, 182–193 (2009).
Dilling, L. & Allredge, A. L. Fragmentation of marine snow by swimming macrozooplankton: A new process impacting carbon cycling in the sea. Deep Sea Res. I 47, 1227–1245 (2000).
Alonso-González, I. J. et al. Role of slowly settling particles in the ocean carbon cycle. Geophys. Res. Lett. 37, L13608 (2010).
Goutx, M. et al. Composition and degradation of marine particles with different settling velocities in the northwestern Mediterranean Sea. Limnol. Oceanogr. 52, 1645–1664 (2007).
Abramson, L., Lee, C., Liu, Z., Wakeham, S. G. & Szlosek, J. Exchange between suspended and sinking particles in the northwest Mediterranean as inferred from the organic composition of in situ pump and sediment trap samples. Limnol. Oceanogr. 55, 725–739 (2010).
Druffel, E. R. M., Bauer, J. E., Griffin, S. & Hwang, J. Penetration of anthropogenic carbon into organic particles of the deep ocean. Geophys. Res. Lett. 30 (2003).
Riley, J. S. et al. The relative contribution of fast and slow sinking particles to ocean carbon export. Global Biogeochem. Cycles 26 (2012).
Karl, D. M., Knauer, G. A. & Martin, J. H. Downward flux of particulate organic matter in the ocean: a particle decomposition paradox. Nature 332, 438–441 (1988).
Cho, B. C. & Azam, F. Major role of bacteria in biogeochemical fluxes in the ocean's interior. Nature 332, 441–443 (1988).
Kellogg, C. T. E. et al. Evidence for microbial attenuation of particle flux in the Amundsen Gulf and Beaufort Sea: elevated hydrolytic enzyme activity on sinking aggregates. Polar Biol. 34, 2007–2023 (2011).
Grossart, H.-P. & Gust, G. Hydrostatic pressure affects physiology and community structure of marine bacteria during settling to 4000 m: an experimental approach. Mar. Ecol. Prog. Ser. 390, 97–104 (2009).
Tamburini, C. et al. Pressure effects on surface Mediterranean prokaryotes and biogenic silica dissolution during a diatom sinking experiments. Aquat. Microb. Ecol. 43, 267–276 (2006).
Hansell, D. A., Carlson, C. A. & Schlitzer, R. Net removal of major marine dissolved organic carbon fractions in the subsurface ocean. Glob. Biogeochem. Cycles 26, GB1016 (2012).
Druffel, E. R. M. & Robison, B. H. Is the deep sea on a diet? Science 284, 1139–1140 (1999).
Druffel, E. R. M. & Williams, P. M. Identification of a deep marine source of particulate organic carbon using bomb 14C. Nature 347, 172–174 (1990).
Lauro, F. M. et al. The genomic basis of trophic strategy in marine bacteria. Proc. Natl Acad. Sci. USA 106, 15527–15533 (2009).
Moeseneder, M. M., Winter, C. & Herndl, G. J. Horizontal and vertical complexity of attached and free-living bacteria of the eastern Mediterranean Sea, determined by 16S rDNA and 16S rRNA fingerprints. Limnol. Oceanogr. 46, 95–107 (2001).
Aristegui, J., Gasol, J. M., Duarte, C. M. & Herndl, G. J., Microbial oceanography of the dark ocean's pelagic realm. Limnol. Oceanogr. 54, 1501–1529 (2009).
Eloe, E. A. et al., Compositional differences in particle-associated and free-living microbial assemblages from an extreme deep-ocean environment. Environ. Microbiol. Rep. 3, 449–458 (2011).
Lauro, F. M. & Bartlett, D. H., Prokaryotic lifestyles in deep sea habitats. Extremophiles 12, 15–25 (2007).
Lauro, F. M., Chastain, R. A., Blankenship, L. E., Yayanos, A. A. & Bartlett, D. H. The unique 16S rRNA genes of piezophiles reflect both phylogeny and adaptation. Appl. Environ. Microbiol. 73, 838–845 (2007).
DeLong, E. F. et al. Community genomics among stratified microbial assemblages in the ocean's interior. Science 311, 496–503 (2006).
Martin-Cuadrado, A-B. et al. Metagenomics of deep Mediterranean, a warm bathypelagic habitat. PLoS One 9, e914 (2007).
Ivars-Martinez, E. et al. Comparative genomics of two ecotypes of the marine planktonic copiotroph Alteromonas macleodii suggests alternative lifestyles associated with different kinds of particulate organic matter. ISME J. 2, 1194–1212 (2008).
Vetter, Y. A., Deming, J. W., Jumars, P. A. & Krieger-Brockett, B. B. A predicitive model of bacterial foraging by means of freely released extracellular enzymes. Microb. Ecol. 36, 75–92 (1998).
Baltar, F. et al. High dissolved extracellular enzymatic activity in the deep central Atlantic Ocean. Aquat. Microb. Ecol. 58, 287–302 (2010).
Antia, A. N. et al. Basin-wide particulate carbon flux in the Atlantic Ocean: regional export patterns and potential for athmospheric CO2 sequestration. Glob. Biogeochem. Cycles 15, 845–862 (2001).
Yokokawa, T., Yang, Y., Motegi, C. & Nagata, T. Large-scale geographical variation in prokaryotic abundance and production in meso- and bathypelagic zones of the central Pacific and Southern Ocean. Limnol. Oceanogr. 58, 61–73 (2013).
Aristegui, J. et al. Dissolved organic carbon support of respiration in the dark ocean. Science 298, 1967 (2002).
Alonso-Saez, L., Galand, P. E., Casamayor, E. O., Pedros-Alio, C. & Bertilsson, S. High bicarbonate assimilation in the dark by Arctic bacteria. ISME J. 4, 1581–1590 (2010).
Swan, B. K. et al. Potential for chemolithoautotrophy among ubiquitous bacteria lineages in the dark ocean. Science 333, 1296–1300 (2011).
Anantharaman, K., Breier, J. A., Sheik, C. S. & Dick, G. J. Evidence for hydrogen oxidation and metabolic plasticity in widespread deep-sea sulfur-oxidizing bacteria. Proc. Natl Acad. Sci. USA 110, 330–335 (2013).
Hügler, M. & Sievert, S. M. Beyond the Calvin cycle: autotrophic carbon fixation in the ocean. Annu. Rev. Mar. Sci. 3, 261–289 (2011).
Azam, F. & Malfatti, F. Microbial structuring of marine ecosystems. Nature Rev. Microbiol. 5, 782–791 (2007).
Reinthaler, T., Aken, H. M.v. & Herndl, G. J. Major contribution of autotrophy to microbial carbon cycling in the deep North Atlantic's interior. Deep Sea Res. II 57, 1572–1580 (2010).
Yakimov, M. M. et al., Contribution of crenarchaeal autotrophic ammonia oxidizers to the dark primary production in Tyrrhenian deep waters (Central Mediterranean Sea). ISME J. 5, 945–961 (2011).
Burd, A. B. et al. Assessing the apparent imbalance between geochemcial and biochemical indicators of meso- and bathypelagic biological activity: What the @$#! is wrong with present calculations of carbon budgets. Deep Sea Res. II 57, 1557–1571 (2010).
Jiao, N. et al. Microbial production of recalcitrant dissolved organic matter: long-term carbon storage in the global ocean. Nature Rev. Microbiol. 8, 593–599 (2010).
Bauer, J. E., Williams, P. M. & Druffel, E. R. M. 14C activity of dissolved organic carbon fractions in the north-central Pacific and Sargasso Sea. Nature 357, 667–670 (1992).
Carlson, C. A. et al. Interactions among dissolved organic carbon, microbial processes, and community structure in the mesopelagic zone of the northwestern Sargasso Sea. Limnol. Oceanogr. 49, 1073–1083 (2004).
Shi, Y., McCarren, J. & Delong, E. F. Transcriptional responses of surface water marine microbial assemblages to deep-sea water amendment. Environ. Microbiol. 14, 191–206 (2012).
Nagata, T. et al. Emerging concepts on microbial processes int he bathypelagic ocean: ecology, biogeochemistry and genomics. Deep Sea Res. II 57, 1519–1536 (2010).
Tamburini, C., Boutrif, M., Garel, M., Colwell, R. R. & Deming, J. W. Prokaryotic responses to hydrostatic pressure in the ocean: a review. Environ. Microbiol. (2013).
Riebesell, U., Körtzinger, A. & Oschlies, A. Sensitivities of marine carbon fluxes to ocean change. Proc. Natl Acad. Sci. USA 106, 20602–20609 (2009).
Acknowledgements
This work was supported by the ERC Advanced Grant MEDEA and the Austrian Science Fund projects: I486-B09 and P23234-B11 to G.J.H. and P23221-B11 to T.R.
Author information
Authors and Affiliations
Contributions
G.J.H. and T.R. contributed equally to this work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Herndl, G., Reinthaler, T. Microbial control of the dark end of the biological pump. Nature Geosci 6, 718–724 (2013). https://doi.org/10.1038/ngeo1921
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ngeo1921
This article is cited by
-
Efficient biological carbon export to the mesopelagic ocean induced by submesoscale fronts
Nature Communications (2024)
-
Increased prokaryotic diversity in the Red Sea deep scattering layer
Environmental Microbiome (2023)
-
Interplay between autotrophic and heterotrophic prokaryotic metabolism in the bathypelagic realm revealed by metatranscriptomic analyses
Microbiome (2023)
-
Top abundant deep ocean heterotrophic bacteria can be retrieved by cultivation
ISME Communications (2023)
-
Phylogenetically and metabolically diverse autotrophs in the world’s deepest blue hole
ISME Communications (2023)