Abstract
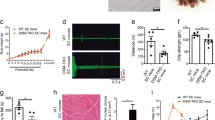
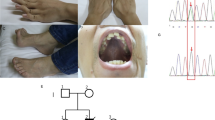
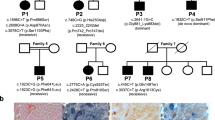
Myotonic dystrophy (DM) results from the amplification of an unstable CTG repeat in the 3′ untranslated region of a transcript encoding a putative serine/threonine kinase. We have analysed the amplification of the repeat and the steady state levels of the DM kinase (DMK) mRNA in tissues and cell lines from normal and congenital DM individuals. Southern blot analysis of DNA samples from a severely affected neonate shows somatic heterogeneity of the repeat in all tissues studied. RNA analyses on these tissues show a marked increase in DMK steady state mRNA levels. We demonstrate that the mutant DMK allele is expressed regardless of the number of CTG repeats and that the increase in DMK mRNA levels is due to elevated mutant mRNA levels. We postulate that elevated DMK levels explains the dominant inheritance pattern of DM.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Vanier, T.M. Dystrophia Myotonia in Childhood. Br. Med. J. 2, 1285–1288 (1960).
Harper, P.S. . in Myotonic Dystrophy 2nd edn (Saunders, Philadelphia, 1989).
Sarnat, H.B. & Silbert, S.W., Maturational Arrest of Fetal Muscle in Neonatal Myotonic Dystrophy. Arch. Neurol. 33, 466–474 (1976).
Soussi-Yanicostas, N. et al. Distinct contractile protein profile in congenital myotonic dystrophy and X-linked myotubular myopathy. Neuromusc Dis 1, 103–111 (1992).
Aslanidis, C. et al. Cloning of the essential myotonic dystrophy region and mapping of the putative defect. Nature 355, 548–551 (1992).
Mahadevan, M. et al. Myotonic dystrophy mutation: an unstable CTG repeat in the 3′ untranslated region of the gene. Science 255, 1253–1255 (1992).
Brook, J.D. et al. Molecular basis of myotonic dystrophy: expansion of a trinucleotide (CTQ) repeat at the 3′ end of a transcript encoding a protein kinase family member. Cell 68, 799–808 (1992).
Buxton, J. et al. Detection of an unstable fragment of DNA specific to individuals with myotonic dystrophy. Nature 355, 547–548 (1992).
Fu, Y.H. et al. An unstable triplet repeat in a gene related to myotonic muscular dystrophy. Science 255, 1256–1258 (1992).
Jansen, G. et al. Characterization of the myotonic dystrophy region predicts multiple protein isoform-encoding mRNAs. Nature Genet. 1, 261–266 (1992).
Harley, H.G. et al. Expansion of an unstable DNA region and phenotypic variation in myotonic dystrophy. Nature 355, 545–546 (1992).
Tsilfidis, C., MacKenzie, A.E., Mettler, G., Barcelo, J. & Korneluk, R.G. Correlation between CTG trinucleotide repeat length and frequency of severe congenital myotonic dystrophy. Nature Genet. 1, 192–195 (1992).
Barcelo, J.M., Mahadevan, M.S., Tsilfidis, C., MacKenzie, A.E. & Korneluk, R.G. Intergenerational stability of the myotonic dystrophy protomutation. Hum. molec. Genet. (in the press)
Caskey, C.T., Pizzuti, A., Fu, Y.-H., Fenwick, R.G. & Nelson, D.L. Triplet repeat mutations in human disease. Science 256, 784–788 (1992).
Richards, R.I. & Sutherland, G.R. Dynamic mutations: A new class of mutations causing human disease. Cell 70, 709–712 (1992).
Howeler, C.J., Busch, H.F.M., Geraedts, J.P.M., Niermeijer, M.F. & Staal, A. Anticipation in myotonic dystrophy: fact or fiction? Brain 112, 779–797 (1989).
Fu, Y.-H. et al. Variation of the CGG repeat at the fragile X site results in genetic instability: Resolution of the Sherman paradox. Cell 67, 1047–1058 (1991).
Annemieke, J.M. et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell 65, 905–914 (1991).
Pieretti, M. et al. Absence of expression of the FMR-1 gene in fragile X syndrome. Cell 66, 817–822 (1991).
Bell, M.V. et al. Physical mapping across the fragile X: Hypermethylation and clinical expression of the fragile X syndrome. Cell 64, 861–866 (1991).
Sutcliffe, J.S. et al. DNA methylation represses FMR-1 transcription in fragile X syndrome. Hum. molec. Genet. 1, 397–400 (1992).
La Spada, A.R., Wilson, E.M., Lubahn, D.B., Harding, A.E. & Fischbeck, K.H. Androgen receptor gene mutations in X-linked spinal and bulbar muscular atrophy. Nature 352, 77–79 (1991).
The Huntington's disease collaborative research group. Cell 72, 971–983 (1993).
McAllister, R.M., Melnyk, J., Finkelstein, J.Z., Adams, E.C. Jr., & Gardner, M.B. Cultivation in vitro of cells derived from a human rhabdomyosarcoma. Cancer 24, 520–526 (1969).
Gililand, G., Perrin, S., Blanchard, K. & Bunn, H.F. Analysis of cytokine mRNA and DNA: detection and quantitation by competitive polymerase chain reaction. Proc. natn. Acad. Sci. U.S.A. 87, 2725–2729 (1990).
Fu, Y.-H. et al. Decreased expression of myotonin-protein kinase messenger RNA and protein in adult form of myotonic dystrophy. Science 260, 235–238 (1993).
Zucker, M. & Stiegler, P. Optimal computer folding of large RNA sequences using thermodynamics and auxiliary information. Nucl. Acids Res. 9, 133–148 (1981).
Mullner, E.W. & Kuhn, L.C. A stem-loop in the 3′ untranslated region mediates iron-dependent regulation of transferrin receptor mRNA stability in the cytoplasm. Cell 53, 815–825 (1988).
Mullner, E.W., Neupert, B. & Kuhn, L.C. A specific mRNA binding factor regulates the iron- dependent stability of cytoplasmic transferrin receptor mRNA. Cell 58, 373–382 (1989).
Peltz, S.W., Brewer, G., Bernstein, P., Hart, P.A. & Ross, J. Regulation of mRNA turnover in eukaryotic cells. Crit. Rev. Euk. Gene Exp. 1, 99–126 (1991).
Bernstein, P.L., Herrick, D.J., Prokipcak, R.D. & Ross, J. Control of c-myc mRNA half-life in vitro by a protein capable of binding to a coding stability determinant. Genes Dev. 6, 642–654 (1992).
Dix, D.J., Lin, P.-N., Kimata, Y. & Theil, E.C. The iron regulatory region of ferritin mRNA is also a positive control elememt for iron-independent translation. Biochemistry 31, 2818–2822 (1992).
Kaspar, R.L., Kakegawa, T., Cranston, H. & White, M.W. A regulatory cis element and a specific binding factor involved in the mitogenic control of murine ribosomal protein L32 translation. J. biol. Chem. 267, 508–514 (1992).
Sengupta, D.N., Berkhout, B., Gatignol, A., Zhou, A. & Silverman, R.H. Direct evidence for translational regulation by leader RNA and Tat protein of human immunodeficiency virus type 1. Proc. natn. Acad. Sci. U.S.A. 87, 7492–7496 (1990).
McCormack, S.J., Thomis, D.C. & Samuel, C.E. Mechanism of interferon action: Identification of a RNA binding domain within the N-terminal region of the human RNA-dependent P1/elF-2 alpha protein kinase. Virology 188, 47–56 (1992).
Wisdom, R. & Lee, W. Translation of c-myc mRNA is required for its post-transcriptional regulation during myogenesis. J. biol. Chem. 265, 19015–19021 (1990).
Taylor, S.S., Buechler, J.A. & Yonemoto, W. cAMP-dependent protein kinase: framework for a diverse family of regulatory enzymes. An. Rev. Biochem. 59, 971–1005 (1990).
Farkas-Bargeton, E. et al. Immaturity of muscle fibers in the congenital form of myotonic dystrophy. J. neurol. Sci. 83, 145–159 (1988).
Li, L., Heller-Harrison, R., Czech, M. & Olson, E. Cyclic AMP-dependent protein kinase inhibits the activity of myogenic helix-loop-helix proteins. Molec. cell. Biol. 12, 4478–4485 (1992).
Li, L. et al. FGF inactivates myogenic helix-loop-helix proteins through phosphorylation of a conserved protein kinase C site in their DNA-binding domain. Cell 71, 1181–1194 (1992).
Piechaczyk, M. et al. Post-transcriptional regulation of glyceraldehyde-3-phosphate-dehydrogenase gene expression in rat tissues. Nucl. Acids Res. 12, 6951–6963 (1984).
Puissant, C. & Houdebine, L.-M. An improvement of the single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Biotechniques 8, 148–149 (1990).
Birnboim, H.C. Rapid extraction of high molecular weight RNA from cultured cells and granulocytes for Northern analysis. Nucl. Acids Res. 16, 1487–1497 (1988).
Sambrook, J., Fritsch, E.F. & Maniatis, T. Molecular Cloning: A Laboratory Manual 2nd edn (Cold Spring Harbor, New York 1989).
Sabourin, L.A. & Hawley, R.G. Suppression of programmed death and G1 arrest in B-cell hybridomas by interleukin-6 is not accompanied by altered expression of immediate early response genes. J. cell. Physiol. 145, 564–574 (1990).
Dretzen, G., Bellard, M., Sassone-Corsi, P. & Chambon, P. A reliable method for the recovery of DNA fragments from agarose and acrylamide gels. Anal. Biochem. 112, 295–298 (1981).
Feinberg, A.P. & Vogelstein, B. A technique for radiolabelling DNA restriction endonuclease fragments to high specific activity. Anal. Biochem. 132, 6–13 (1983).
Mahadevan, M. et al. Structure and genomic sequence of the myotonic dystrophy (DM kinase) gene. Hum. molec. Genet. (in the press).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sabouri, L., Mahadevan, M., Narang, M. et al. Effect of the myotonic dystrophy (DM) mutation on mRNA levels of the DM gene. Nat Genet 4, 233–238 (1993). https://doi.org/10.1038/ng0793-233
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ng0793-233
This article is cited by
-
CTCF-binding sites flank CTG/CAG repeats and form a methylation-sensitive insulator at the DM1 locus
Nature Genetics (2001)
-
Trinucleotide repeat expansion at the myotonic dystrophy locus reduces expression of DMAHP
Nature Genetics (1997)
-
Dodecamer repeat expansion in cystatin B gene in progressive myoclonus epilepsy
Nature (1997)