Abstract
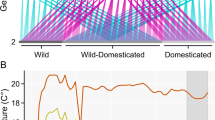
Pacific salmon provide critical sustenance for millions of people worldwide and have far-reaching impacts on the productivity of ecosystems. Rising temperatures now threaten the persistence of these important fishes1,2, yet it remains unknown whether populations can adapt. Here, we provide the first evidence that a Pacific salmon has both physiological and genetic capacities to increase its thermal tolerance in response to rising temperatures. In juvenile chinook salmon (Oncorhynchus tshawytscha), a 4 °C increase in developmental temperature was associated with a 2 °C increase in key measures of the thermal performance of cardiac function3,4. Moreover, additive genetic effects significantly influenced several measures of cardiac capacity, indicative of heritable variation on which selection can act. However, a lack of both plasticity and genetic variation was found for the arrhythmic temperature of the heart, constraining this upper thermal limit to a maximum of 24.5 ± 2.2 °C. Linking this constraint on thermal tolerance with present-day river temperatures and projected warming scenarios5, we predict a 17% chance of catastrophic loss in the population by 2100 based on the average warming projection, with this chance increasing to 98% in the maximum warming scenario. Climate change mitigation is thus necessary to ensure the future viability of Pacific salmon populations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Farrell, A. P. et al. Pacific salmon in hot water: Applying aerobic scope models and biotelemetry to predict the success of spawning migrations. Phys. Biochem. Zool. 81, 697–708 (2008).
Crozier, L. G., Zabel, R. W. & Hamlett, A. F. Predicting differential effects of climate change at the population level with life-cycle models of spring chinook salmon. Glob. Change Biol. 14, 236–249 (2008).
Casselman, M. T., Anttila, K. & Farrell, A. P. Using maximum heart rate as a rapid screening tool to determine optimum temperature for aerobic scope in Pacific salmon Oncorhynchus spp. J. Fish Biol. 80, 358–377 (2012).
Anttila, K., Casselman, M. T., Schulte, P. M. & Farrell, A. P. Optimum temperature in juvenile salmonids: Connecting subcellular indicators to tissue function and whole-organism thermal optimum. Physiol. Biochem. Zool. 86, 245–256 (2013).
Christensen, J. H. et al. in Climate Change 2013: The Physical Science Basis (eds Stocker, T. F. et al.) 1217–1308 (Cambridge Univ. Press, 2013).
Dillon, M. E., Wang, G. & Huey, R. B. Global metabolic impacts of recent climate warming. Nature 467, 704–706 (2010).
Angilletta, M. J. Thermal Adaptation: A Theoretical and Empirical Synthesis (Oxford Univ. Press, 2009).
Somero, G. N. The physiology of climate change: How potentials for acclimatization and genetic adaptation will determine ‘winners’ and ‘losers’. J. Exp. Biol. 213, 912–920 (2010).
Crozier, L. G. & Hutchings, J. A. Plastic and evolutionary responses to climate change in fish. Evol. Appl. 7, 68–87 (2014).
Munday, P. L., Warner, R. R., Monro, K., Pandolfi, J. M. & Marshall, D. J. Predicting evolutionary responses to climate change in the sea. Ecol. Lett. 16, 1488–1500 (2013).
Pörtner, H. O. & Knust, R. Climate change affects marine fishes through the oxygen limitation of thermal tolerance. Science 315, 95–97 (2007).
Pörtner, H. O. & Farrell, A. P. Physiology and climate change. Science 322, 690–692 (2008).
Clark, T. D., Sandblom, E. & Jutfelt, F. Aerobic scope measurements of fishes in an era of climate change: Respirometry, relevance and recommendations. J. Exp. Biol. 216, 2771–2782 (2013).
Clark, T. D., Sandblom, E. & Jutfelt, F. Response to Farrell and to Pörtner and Giomi. J. Exp. Biol. 216, 4495–4497 (2013).
Farrell, A. P. Environment, antecedents and climate change: Lessons from the study of temperature physiology and river migration of salmonids. J. Exp. Biol. 212, 3771–3780 (2009).
Logan, C. A., Kost, L. E. & Somero, G. N. Latitudinal differences in Mytilus californianus thermal physiology. Mar. Ecol. Prog. Ser. 450, 93–105 (2012).
Iftikar, F. I. et al. Could thermal sensitivity of mitochondria determine species distribution in a changing climate? J. Exp. Biol. 217, 2348–2357 (2014).
Weng, K. C. et al. Satellite tagging and cardiac physiology reveal niche expansion in salmon sharks. Science 310, 104–106 (2005).
Eliason, E. J. et al. Differences in thermal tolerance among sockeye salmon populations. Science 332, 109–112 (2011).
Crozier, L. G. & Zabel, R. W. Climate impacts at multiple scales: Evidence for differential population responses in juvenile chinook salmon. J. Anim. Ecol. 75, 1100–1109 (2006).
Lynch, M. & Walsh, B. Genetics and Analysis of Quantitative Traits (Sinauer Associates, 1998).
Chen, Z. et al. Optimum and maximum temperatures of sockeye salmon (Oncorhynchus nerka) populations hatched at different temperatures. Can. J. Zool. 91, 265–274 (2013).
Anttila, K. et al. Atlantic salmon show capability for cardiac acclimation to warm temperatures. Nature Commun. 5, 4252 (2014).
Heath, D. D., Heath, J. W., Bryden, C. A., Johnson, R. M. & Fox, C. W. Rapid evolution of egg size in captive salmon. Science 299, 1738–1740 (2003).
Muñoz, N. J. et al. Indirect genetic effects underlie oxygen-limited thermal tolerance within a coastal population of chinook salmon. Proc. R. Soc. B 281, 20141082 (2014).
Shiels, H. A. & Farrell, A. P. The effect of temperature and adrenaline on the relative importance of the sarcoplasmic reticulum in contributing Ca2+ to force development in isolated ventricular trabeculae from rainbow trout. J. Exp. Biol. 200, 1607–1621 (1997).
Castilho, P. C., Landeira-Fernandez, A. M., Morrissette, J. & Block, B. A. Elevated Ca2+ ATPase (SERCA2) activity in tuna hearts: Comparative aspects of temperature dependence. Comp. Biochem. Physiol. 148, 124–132 (2007).
Jensen, L. F. et al. Local adaptation in brown trout early life-history traits: Implications for climate change adaptability. Proc. R. Soc. B 275, 2859–2868 (2008).
Meier, K. et al. Local adaptation at the transcriptome level in brown trout: Evidence from early life-history temperature genomic reaction norms. PLoS ONE 9, e85171 (2014).
Donelson, J. M., Munday, P. L., McCormick, M. I. & Pitcher, C. R. Rapid transgenerational acclimation of a tropical reef fish to climate change. Nature Clim. Change 2, 30–32 (2012).
Acknowledgements
We thank A. Heath and the staff at Yellow Island Aquaculture Ltd for their support with fish husbandry, A. Berchtold for assisting with the heart rate experiment, D. MacKinlay and the staff at the Fisheries and Oceans Canada Quinsam River salmon hatchery for their help with gamete collection, and S. Garner for help with the climate change susceptibility model. This study was supported by Discovery grants to B.D.N. and A.P.F. from the Natural Science and Engineering Research Council of Canada. A.P.F. holds a Canada Research Chair in Fish Physiology, Culture and Conservation. All experiments followed ethical guidelines from the Canadian Council on Animal Care as reviewed and approved by the Animal Use Subcommittees at the University of Western Ontario (protocol no. 2010-214) and the University of British Columbia (protocol no. 810-022).
Author information
Authors and Affiliations
Contributions
All authors designed the experiment; N.J.M. conducted the experiment and data analyses; J.W.H. contributed materials and logistical support during the experiment; N.J.M., A.P.F. and B.D.N. wrote the paper. All authors provided intellectual input, and read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Muñoz, N., Farrell, A., Heath, J. et al. Adaptive potential of a Pacific salmon challenged by climate change. Nature Clim Change 5, 163–166 (2015). https://doi.org/10.1038/nclimate2473
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nclimate2473
This article is cited by
-
Compound marine heatwaves and ocean acidity extremes
Nature Communications (2022)
-
Genetic variation for upper thermal tolerance diminishes within and between populations with increasing acclimation temperature in Atlantic salmon
Heredity (2021)
-
One size does not fit all: variation in thermal eco-physiology among Pacific salmonids
Reviews in Fish Biology and Fisheries (2021)
-
Genomic signatures and correlates of widespread population declines in salmon
Nature Communications (2019)
-
A global synthesis of biodiversity responses to glacier retreat
Nature Ecology & Evolution (2019)