Abstract
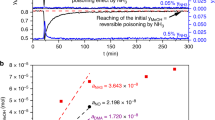
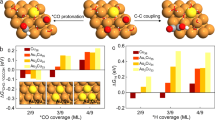
Industrial hydrogen production through methane steam reforming exceeds 50 million tons annually and accounts for 2–5% of global energy consumption. The hydrogen product, even after processing by the water–gas shift, still typically contains ∼1% CO, which must be removed for many applications. Methanation (CO + 3H2 → CH4 + H2O) is an effective solution to this problem, but consumes 5–15% of the generated hydrogen. The preferential oxidation (PROX) of CO with O2 in hydrogen represents a more-efficient solution. Supported gold nanoparticles, with their high CO-oxidation activity and notoriously low hydrogenation activity, have long been examined as PROX catalysts, but have shown disappointingly low activity and selectivity. Here we show that, under the proper conditions, a commercial Au/Al2O3 catalyst can remove CO to below 10 ppm and still maintain an O2-to-CO2 selectivity of 80–90%. The key to maximizing the catalyst activity and selectivity is to carefully control the feed-flow rate and maintain one to two monolayers of water (a key CO-oxidation co-catalyst) on the catalyst surface.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Neef, H. J. International overview of hydrogen and fuel cell research. Energy 34, 327–333 (2009).
Liu, K., Song, C. & Subramani, V. Hydrogen and Syngas Production and Purification Technologies (John Wiley & Sons, Inc., 2010).
Ghenciu, A. F. in Fuel Cells Compendium (eds Brandon, N. & Thompsett, D.) Ch. 5, 91–106 (Elsevier, 2005).
European Commission Best Available Techniques for the Manufacture of Large Volume Inorganic Chemicals – Ammonia, Acids and Fertilisers (European Commission, 2007).
Spath, P. L. & Mann, M. K. Life Cycle Assessment of Hydrogen Production via Natural Gas Steam Reforming (Department of Energy, National Renewable Energy Laboratory, 2001).
Greenhouse Gas Emissions Calculations and References (US Environment Protection Agency, 2015); www.epa.gov/energy/ghg-equivalencies-calculator-calculations-and-references
Landon, P. et al. Selective oxidation of CO in the presence of H2, H2O and CO2 utilising Au/α-Fe2O3 catalysts for use in fuel cells. J. Mater. Chem. 16, 199–208 (2006).
Schumacher, B., Denkwitz, Y., Plzak, V., Kinne, M. & Behm, R. J. Kinetics, mechanism, and the influence of H2 on the CO oxidation reaction on a Au/TiO2 catalyst. J. Catal. 224, 449–462 (2004).
Lakshmanan, P., Park, J. & Park, E. Recent advances in preferential oxidation of CO in H2 over gold catalysts. Catal. Surv. Asia 18, 75–88 (2014).
Green, I. X., Tang, W., Neurock, M. & Yates, J. T. Jr . Spectroscopic observation of dual catalytic sites during oxidation of CO on a Au/TiO2 catalyst. Science 333, 736–739 (2011).
Saavedra, J., Doan, H. A., Pursell, C. J., Grabow, L. C. & Chandler, B. D. The critical role of water at the gold–titania interface in catalytic CO oxidation. Science 345, 1599–1602 (2014).
Valden, M., Lai, X. & Goodman, D. W. Onset of catalytic activity of gold clusters on titania with the appearance of nonmetallic properties. Science 281, 1647–1650 (1998).
Bond, G. C., Louis, C. & Thompson, D. T. Catalysis by Gold Vol. 6 (Imperial College Press, 2006).
Avgouropoulos, G. et al. A comparative study of Pt/γ-Al2O3, Au/α-Fe2O3 and CuO–CeO2 catalysts for the selective oxidation of carbon monoxide in excess hydrogen. Catal. Today 75, 157–167 (2002).
Grisel, R. J. H. & Nieuwenhuys, B. E. Selective oxidation of CO, over supported Au catalysts. J. Catal. 199, 48–59 (2001).
Ivanova, S., Pitchon, V., Petit, C. & Caps, V. Support effects in the gold-catalyzed preferential oxidation of CO. ChemCatChem 2, 556–563 (2010).
Widmann, D., Liu, Y., Schueth, F. & Behm, R. J. Support effects in the Au-catalyzed CO oxidation—correlation between activity, oxygen storage capacity, and support reducibility. J. Catal. 276, 292–305 (2010).
Tompos, A. et al. Role of modifiers in multi-component MgO-supported Au catalysts designed for preferential CO oxidation. J. Catal. 266, 207–217 (2009).
Liu, Y. et al. Three-dimensionally ordered macroporous Au/CeO2–Co3O4 catalysts with mesoporous walls for enhanced CO preferential oxidation in H2-rich gases. J. Catal. 296, 65–76 (2012).
Li, X. et al. Activation and deactivation of Au-Cu/SBA-15 catalyst for preferential oxidation of CO in H2-rich gas. ACS Catal. 2, 360–369 (2012).
Kim, W. B., Voitl, T., Rodriguez-Rivera, G. J., Evans, S. T. & Dumesic, J. A. Preferential oxidation of CO in H2 by aqueous polyoxometalates over metal catalysts. Angew. Chem. Int. Ed. 44, 778–782 (2005).
Carrettin, S., Concepcion, P., Corma, A., Lopez Nieto, J. M. & Puntes, V. F. Gold catalysts: nanocrystalline CeO2 increases the activity of Au for CO oxidation by two orders of magnitude. Angew. Chem. Int. Ed. 43, 2538–2540 (2004).
Cargnello, M. et al. Active and stable embedded Au@CeO2 catalysts for preferential oxidation of CO. Chem. Mater. 22, 4335–4345 (2010).
Singh, J. A., Overbury, S. H., Dudney, N. J., Li, M. & Veith, G. M. Gold nanoparticles supported on carbon nitride: influence of surface hydroxyls on low temperature carbon monoxide oxidation. ACS Catal. 2, 1138–1146 (2012).
Ketchie, W. C., Murayama, M. & Davis, R. J. Promotional effect of hydroxyl on the aqueous phase oxidation of carbon monoxide and glycerol over supported Au catalysts. Top. Catal. 44, 307–317 (2007).
Ide, M. S. & Davis, R. J. The important role of hydroxyl on oxidation catalysis by gold nanoparticles. Acc. Chem. Res. 47, 825–833 (2014).
Date, M. & Haruta, M. Moisture effect on CO oxidation over Au/TiO2 catalyst. J. Catal. 201, 221–224 (2001).
Date, M., Okumura, M., Tsubota, S. & Haruta, M. Vital role of moisture in the catalytic activity of supported gold nanoparticles. Angew. Chem. Int. Ed. 43, 2129–2132 (2004).
Calla, J. T. & Davis, R. J. Oxygen-exchange reactions during CO oxidation over titania- and alumina-supported Au nanoparticles. J. Catal. 241, 407–416 (2006).
Fujitani, T. & Nakamura, I. Mechanism and active sites of the oxidation of CO over Au/TiO2 . Angew. Chem. Int. Ed. 50, 10144–10147 (2011).
Ojeda, M., Zhan, B.-Z. & Iglesia, E. Mechanistic interpretation of CO oxidation turnover rates on supported Au clusters. J. Catal. 285, 92–102 (2012).
Widmann, D. & Behm, R. J. Activation of molecular oxygen and the nature of the active oxygen species for CO oxidation on oxide supported Au catalysts. Acc. Chem. Res. 47, 740–749 (2014).
Kung, M. C., Davis, R. J. & Kung, H. H. Understanding Au-catalyzed low-temperature CO oxidation. J. Phys. Chem. C 111, 11767–11775 (2007).
Haruta, M. Spiers Memorial Lecture. Role of perimeter interfaces in catalysis by gold nanoparticles. Faraday Discuss. 152, 11–32 (2011).
Widmann, D., Hocking, E. & Behm, R. J. On the origin of the selectivity in the preferential CO oxidation on Au/TiO2—nature of the active oxygen species for H2 oxidation. J. Catal. 317, 272–276 (2014).
Fujitani, T., Nakamura, I., Akita, T., Okumura, M. & Haruta, M. Hydrogen dissociation by gold clusters. Angew. Chem. Int. Ed. 48, 9515–9518 (2009).
Cargnello, M. et al. Control of metal nanocrystal size reveals metal–support interface role for ceria catalysts. Science 341, 771–773 (2013).
Kotobuki, M., Leppelt, R., Hansgen, D. A., Widmann, D. & Behm, R. J. Reactive oxygen on a Au/TiO2 supported catalyst. J. Catal. 264, 67–76 (2009).
Schubert, M. M., Venugopal, A., Kahlich, M. J., Plzak, V. & Behm, R. J. Influence of H2O and CO2 on the selective CO oxidation in H2-rich gases over Au/α-Fe2O3 . J. Catal. 222, 32–40 (2004).
Saavedra, J., Powell, C., Panthi, B., Pursell, C. J. & Chandler, B. D. CO oxidation over Au/TiO2 catalyst: pretreatment effects, catalyst deactivation, and carbonates production. J. Catal. 307, 37–47 (2013).
Denkwitz, Y. et al. Stability and deactivation of unconditioned Au/TiO2 catalysts during CO oxidation in a near-stoichiometric and O2-rich reaction atmosphere. J. Catal. 251, 363–373 (2007).
Quinet, E. et al. On the mechanism of hydrogen-promoted gold-catalyzed CO oxidation. J. Catal. 268, 384–389 (2009).
Carrasco, J., Hodgson, A. & Michaelides, A. A molecular perspective of water at metal interfaces. Nature Mater. 11, 667–674 (2012).
Gong, J. Structure and surface chemistry of gold-based model catalysts. Chem. Rev. 112, 2987–3054 (2011).
Ikemiya, N. & Gewirth, A. A. Initial stages of water adsorption on Au surfaces. J. Am. Chem. Soc. 119, 9919–9920 (1997).
Pursell, C. J., Hartshorn, H., Ward, T., Chandler, B. D. & Boccuzzi, F. Application of the Temkin model to the adsorption of CO on gold. J. Phys. Chem. C 115, 23880–23892 (2011).
Guzman, J., Carrettin, S. & Corma, A. Spectroscopic evidence for the supply of reactive oxygen during CO oxidation catalyzed by gold supported on nanocrystalline CeO2 . J. Am. Chem. Soc. 127, 3286–3287 (2005).
Bus, E., Miller, J. T. & van Bokhoven, J. A. Hydrogen chemisorption on Al2O3-supported gold catalysts. J. Phys. Chem. B 109, 14581–14587 (2005).
Hartshorn, H., Pursell, C. J. & Chandler, B. D. Adsorption of CO on supported gold nanoparticle catalysts: a comparative study. J. Phys. Chem. C 113, 10718–10725 (2009).
Acknowledgements
The authors kindly thank J. Kenvin (Micromeritics Instrument Corporation) and S.M.K. Shahri (Pennsylvania State University) for assistance with the measurement of the water-adsorption isotherms. The authors gratefully acknowledge the US National Science Foundation (Grant No CBET-1160217 and No. CHE-1012395) for financial support of this work. Z.C. and R.M.R. acknowledge the Department of Energy, Office of Basic Energy Sciences, Chemical Sciences, Geosciences, and Biosciences Division, Catalysis Sciences Program under grant No. DE-FG02-12ER16364 for partial funding of this research.
Author information
Authors and Affiliations
Contributions
J.S., B.D.C. and C.J.P. designed the catalysis experiments; J.S. performed the catalysis experiments and analysed the data; B.D.C. and C.J.P. designed the infrared spectroscopy experiments; T.W. performed the infrared spectroscopy experiments and analysed the data; B.D.C. and R.M.R. designed the gas-adsorption experiments and analysed the data; Z.C. and R.M.R. designed and performed the X-ray photoemission spectroscopy and X-ray diffraction experiments (see the Supplementary Information) and analysed the data; J.S., B.D.C., R.M.R. and C.J.P. co-wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 868 kb)
Rights and permissions
About this article
Cite this article
Saavedra, J., Whittaker, T., Chen, Z. et al. Controlling activity and selectivity using water in the Au-catalysed preferential oxidation of CO in H2. Nature Chem 8, 584–589 (2016). https://doi.org/10.1038/nchem.2494
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2494
This article is cited by
-
Recent advances on catalysts for preferential oxidation of CO
Nano Research (2023)
-
Effects of water adsorption on active site-dependent H2 activation over MgO nanoflakes
Nano Research (2023)
-
Titanium-doped Boron Nitride Fullerenes as Novel Single-atom Catalysts for CO Oxidation
Catalysis Letters (2022)
-
Atomically dispersed iron hydroxide anchored on Pt for preferential oxidation of CO in H2
Nature (2019)
-
A highly CO-tolerant atomically dispersed Pt catalyst for chemoselective hydrogenation
Nature Nanotechnology (2019)