Abstract
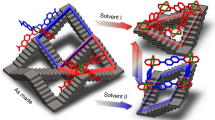
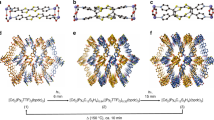
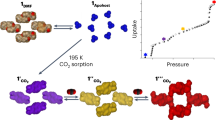
The fabrication of porous coordination frameworks in thin-film forms has been investigated intensively with a view to using their structural response to external stimuli and guests for potential nanotechnological applications, for example as membranes for gas separation. Here we report a coordination framework that exhibits a dynamic guest-sorption behaviour in a nanometre-sized thin-film form (16 nm thick), yet shows no guest uptake in the bulk. Highly oriented crystalline thin films of this coordination framework—which consists of interdigitated two-dimensional layers of {Fe(py)2[Pt(CN)4]} (py, pyridine)—were fabricated through liquid-phase layer-by-layer synthesis. The resulting thin film exhibited a clear guest uptake with a structural transformation of the gate-opening type as characterized by in situ X-ray diffraction. Increasing the film's thickness markedly suppressed this behaviour. We envisage that such a crystal-downsizing effect may be observed with other coordination frameworks, and may be of use to develop functional materials, for example, for switching or sensing devices.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Blossey, R. Self-cleaning surfaces—virtual realities. Nature Mater. 2, 301–306 (2003).
Parker, A. R., Welch, V. L., Driver, D. & Martini, N. Structural colour: opal analogue discovered in a weevil. Nature 426, 786–787 (2003).
Hilf, R. J. C. & Dutzler, R. Structure of a potentially open state of a proton-activated pentameric ligand-gated ion channel. Nature 457, 115–118 (2009).
Kato, H. E. et al. Crystal structure of the channelrhodopsin light-gated cation channel. Nature 482, 369–374 (2012).
Long, S. B., Campbell, E. B. & MacKinnon, R. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science 309, 897–903 (2005).
Green, J. E. et al. A 160-kilobit molecular electronic memory patterned at 1011 bits per square centimetre. Nature 445, 414–417 (2007).
Tokarev, I. & Minko, S. Stimuli-responsive hydrogel thin films. Soft Matter 5, 511–524 (2009).
Mendes, P. M. Stimuli-responsive surfaces for bio-applications. Chem. Soc. Rev. 37, 2512–2529 (2008).
Cohen Stuart, M. A. et al. Emerging applications of stimuli-responsive polymer materials. Nature Mater. 9, 101–113 (2010).
Yaghi, O. M. et al. Reticular synthesis and the design of new materials. Nature 423, 705–714 (2003).
Férey, G. Hybrid porous solids: past, present, future. Chem. Soc. Rev. 37, 191–214 (2008).
Kitagawa, S., Kitaura, R. & Noro, S. Functional porous coordination polymers. Angew. Chem. Int. Ed. 43, 2334–2375 (2004).
Yamada, T., Otsubo, K., Makiura, R. & Kitagawa, H. Designer co-ordination polymers: dimensional crossover architectures and proton conduction. Chem. Soc. Rev. 42, 6655–6669 (2013).
Furukawa, H., Cordova, K. E., O'Keeffe, M. & Yaghi, O. M. The chemistry and applications of metal–organic frameworks. Science 341, 1230444 (2013).
Furukawa, S., Reboul, J., Diring, S., Sumida, K. & Kitagawa, S. Structuring of metal–organic frameworks at the mesoscopic/macroscopic scale. Chem. Soc. Rev. 43, 5700–5734 (2014).
Kole, G. K. & Vittal, J. J. Solid-state reactivity and structural transformations involving coordination polymers. Chem. Soc. Rev. 42, 1755–1775 (2012).
Chae, H. K. et al. A route to high surface area, porosity and inclusion of large molecules in crystals. Nature 427, 523–527 (2004).
Sadakiyo, M., Yamada, T. & Kitagawa, H. Rational designs for highly proton-conductive metal–organic frameworks. J. Am. Chem. Soc. 131, 9906–9907 (2009).
Yoon, M., Srirambalaji, R. & Kim, K. Homochiral metal–organic frameworks for asymmetric heterogeneous catalysis. Chem. Rev. 112, 1196–1231 (2012).
Horike, S., Shimomura, S. & Kitagawa, S. Soft porous crystals. Nature Chem. 1, 695–704 (2009).
Serre, C. et al. Very large breathing effect in the first nanoporous chromium(III)-based solids: MIL-53 or CrIII(OH)·{O2C–C6H4–CO2}·{HO2C–C6H4–CO2H}x·H2Oy . J. Am. Chem. Soc. 124, 13519–13526 (2002).
Kitaura, R., Seki, K., Akiyama, G. & Kitagawa, S. Porous coordination-polymer crystals with gated channels specific for supercritical gases. Angew. Chem. Int. Ed. 42, 428–431 (2003).
Matsuda, R. et al. Guest-shape responsive fitting of porous coordination polymer with shrinkable framework. J. Am. Chem. Soc. 126, 14063–14070 (2004).
Chen, B. L. et al. A microporous metal–organic framework for gas chromatographic separation of alkanes. Angew. Chem. Int. Ed. 45, 1390–1393 (2006).
Bétard, A. & Fischer, R. A. Metal–organic framework thin films: from fundamentals to applications. Chem. Rev. 112, 1055–1083 (2012).
Shekhah, O., Liu, J., Fischer, R. A. & Wöll, C. MOF thin films: existing and future applications. Chem. Soc. Rev. 40, 1081–1106 (2011).
Zacher, D., Schmid, R., Wöll, C. & Fischer, R. A. Surface chemistry of metal–organic frameworks at the liquid–solid interface. Angew Chem. Int. Ed. 50, 176–199 (2011).
Otsubo, K. & Kitagawa, H. Metal–organic framework thin films with well-controlled growth directions confirmed by X-ray study. APL Mater. 2, 124105 (2014).
Zhuang, J. L., Terfort, A. & Wöll, C. Formation of oriented and patterned films of metal–organic frameworks by liquid phase epitaxy: a review. Coord. Chem. Rev. 307, 391–424 (2016).
Shekhah, O. et al. Step-by-step route for the synthesis of metal–organic frameworks. J. Am. Chem. Soc. 129, 15118–15119 (2007).
Kanaizuka, K. et al. Construction of highly oriented crystalline surface coordination polymers composed of copper dithiooxamide complexes. J. Am. Chem. Soc. 130, 15778–15779 (2008).
Arslan, H. K. et al. Intercalation in layered metal–organic frameworks: reversible inclusion of an extended π-system. J. Am. Chem. Soc. 133, 8158–8161 (2011).
Liu, B. et al. Enantiopure metal–organic framework thin films: oriented SURMOF growth and enantioselective adsorption. Angew. Chem. Int. Ed. 51, 807–810 (2012).
Otsubo, K., Haraguchi, T., Sakata, O., Fujiwara, A. & Kitagawa, H. Step-by-step fabrication of a highly oriented crystalline three-dimensional pillared-layer-type metal–organic framework thin film confirmed by synchrotron X-ray diffraction. J. Am. Chem. Soc. 134, 9605–9608 (2012).
Haraguchi, T., Otsubo, K., Sakata, O., Fujiwara, A. & Kitagawa, H. Remarkable lattice shrinkage in highly oriented crystalline three-dimensional metal–organic framework thin films. Inorg. Chem. 54, 11593–11595 (2015).
Makiura, R. et al. Surface nano-architecture of a metal–organic framework. Nature Mater. 9, 565–571 (2010).
Motoyama, S., Makiura, R., Sakata, O. & Kitagawa, H. Highly crystalline nanofilm by layering of porphyrin metal–organic framework sheets. J. Am. Chem. Soc. 133, 5640–5643 (2011).
Gang, X., Yamada, T., Otsubo, K., Sakaida, S. & Kitagawa, H. Facile “modular assembly” for fast construction of a highly oriented crystalline MOF nanofilm. J. Am. Chem. Soc. 134, 16524–16527 (2012).
Scherb, C., Koehn, R. & Bein, T. Sorption behavior of an oriented surface-grown MOF-film studied by in situ X-ray diffraction. J. Mater. Chem. 20, 3046–3051 (2010).
Lee, H. J., Cho, W., Jung, S. & Oh, M. Morphology-selective formation and morphology-dependent gas-adsorption properties of coordination polymer particles. Adv. Mater. 21, 674–677 (2009).
Farha, O. K. et al. Gas-sorption properties of cobalt(II)–carborane-based coordination polymers as a function of morphology. Small 5, 1727–1731 (2009).
Horcajada, P. et al. Colloidal route for preparing optical thin films of nanoporous metal–organic frameworks. Adv. Mater. 21, 1931–1935 (2009).
Tanaka, D. et al. Rapid preparation of flexible porous coordination polymer nanocrystals with accelerated guest adsorption kinetics. Nature Chem. 2, 410–416 (2010).
Sakata, Y. et al. Shape-memory nanopores induced in coordination frameworks by crystal downsizing. Science 339, 193–196 (2013).
Peng, H. et al. Re-appearance of cooperativity in ultra-small spin-crossover [Fe(pz){Ni(CN)4}] nanoparticles. Angew. Chem. Int. Ed. 53, 10894–10898 (2014).
Hofmann, K. A. & Küspert, F. Verbindungen von Kohlenwasserstoffen mit Metallsalzen. Z. Anorg. Chem. 15, 204–207 (1897).
Powell, H. M. & Rayner, J. H. Clathrate compound formed by benzene with an ammonia–nickel cyanide complex. Nature 163, 566–567 (1949).
Kitazawa, T. et al. Spin-crossover behaviour of the coordination polymer FeII(C5H5N)2NiII(CN)4 . J. Mater. Chem. 6, 119–121 (1996).
Niel, V., Martínez-Agudo, J. M., Muñoz, M. C., Gasper, A. B. & Real, J. A. Cooperative spin crossover behavior in cyanide-bridged Fe(II)–M(II) bimetallic 3D Hofmann-like networks (M = Ni, Pd, and Pt). Inorg. Chem. 40, 3838–3839 (2001).
Ohba, M. et al. Bidirectional chemo-switching of spin state in a microporous framework. Angew. Chem. Int. Ed. 48, 4767–4771 (2009).
Acknowledgements
This work was partly supported by Grants-in-Aid for Scientific Research (A) (Grant No. 20350030 and 23245012) and Grant-in-Aid for Young Scientists (B) (Grant No. 25810039) from the Japan Society for the Promotion of Science. Synchrotron XRD measurements were supported by JASRI (Proposal No. 2010B1468, 2011A1382, 2011A1463, 2011B1013, 2011B1529, 2012A1505, 2012A1508, 2012B1518, 2012B1304, 2013A1146, 2013A1486 and 2013B1410).
Author information
Authors and Affiliations
Contributions
K.O. and H.K. conceived the work and designed this study. S.S., K.O. and C.S. performed the experiments. O.S., C.S., A.F. and M.T. contributed to the synchrotron XRD measurements. S.S., K.O. and H.K. co-wrote the manuscript. All the authors discussed and commented on the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 7447 kb)
Supplementary information
Crystallographic data for the bulk-1 structure. (CIF 157 kb)
Rights and permissions
About this article
Cite this article
Sakaida, S., Otsubo, K., Sakata, O. et al. Crystalline coordination framework endowed with dynamic gate-opening behaviour by being downsized to a thin film. Nature Chem 8, 377–383 (2016). https://doi.org/10.1038/nchem.2469
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2469
This article is cited by
-
Isoconversional analysis of thermally stimulated events on pillared cyanometallates
Journal of Thermal Analysis and Calorimetry (2024)
-
Reversible transformations between the non-porous phases of a flexible coordination network enabled by transient porosity
Nature Chemistry (2023)
-
Mechanistic insights into the deformation and degradation of a 2D metal organic framework
npj 2D Materials and Applications (2023)
-
Metal-organic layers: Preparation and applications
Science China Materials (2023)
-
A spin-crossover framework endowed with pore-adjustable behavior by slow structural dynamics
Nature Communications (2022)