Abstract
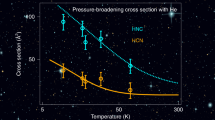
The prototypical F + H2 → HF + H reaction possesses a substantial energetic barrier (~800 K) and might therefore be expected to slow to a negligible rate at low temperatures. It is, however, the only source of interstellar HF, which has been detected in a wide range of cold (10–100 K) environments. In fact, the reaction does take place efficiently at low temperatures due to quantum-mechanical tunnelling. Rate constant measurements at such temperatures have essentially been limited to fast barrierless reactions, such as those between two radicals. Using uniform supersonic hydrogen flows we can now report direct experimental measurements of the rate of this reaction down to a temperature of 11 K, in remarkable agreement with state-of-the-art quantum reactive scattering calculations. The results will allow a stronger link to be made between observations of interstellar HF and the abundance of the most common interstellar molecule, H2, and hence a more accurate estimation of the total mass of astronomical objects.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Levine, R. D. & Bernstein, R. B. Molecular Reaction Dynamics and Chemical Reactivity (Oxford Univ. Press, 1987).
Neumark, D. M., Wodtke, A. M., Robinson, G. N., Hayden, C. C. & Lee, Y. T. Molecular-beam studies of the F + H2 reaction. J. Chem. Phys. 82, 3045–3066 (1985).
Skodje, R. T. et al. Resonance-mediated chemical reaction: F +HD → HF + D. Phys. Rev. Lett. 85, 1206–1209 (2000).
Qiu, M. H. et al. Observation of Feshbach resonances in the F + H2 → HF + H reaction. Science 311, 1440–1443 (2006).
Che, L. et al. Breakdown of the Born–Oppenheimer approximation in the F + o-D2 → DF + D reaction. Science 317, 1061–1064 (2007).
Althorpe, S. C. Setting the trap for reactive resonances. Science 327, 1460–1461 (2010).
Stark, K. & Werner, H. J. An accurate multireference configuration interaction calculation of the potential energy surface for the F + H2 → HF + H reaction. J. Chem. Phys. 104, 6515–6530 (1996).
Li, G., Werner, H-J., Lique, F. & Alexander, M. H. New ab initio potential energy surfaces for the F + H2 reaction. J. Chem. Phys. 127, 174302 (2007).
Alexander, M. H., Manolopoulos, D. E. & Werner, H. J. An investigation of the F + H2 reaction based on a full ab initio description of the open-shell character of the F(2P) atom. J. Chem. Phys. 113, 11084–11100 (2000).
Aquilanti, V. et al. Benchmark rate constants by the hyperquantization algorithm. The F + H2 reaction for various potential energy surfaces: features of the entrance channel and of the transition state, and low temperature reactivity. Chem. Phys. 308, 237–253 (2005).
Manolopoulos, D. E. The dynamics of the F + H2 reaction. J. Chem. Soc. Faraday Trans. 93, 673–683 (1997).
Lique, F. et al. Evidence for excited spin–orbit state reaction dynamics in F + H2: theory and experiment. J. Chem. Phys. 128, 084313 (2008).
Neufeld, D. A., Zmuidzinas, J., Schilke, P. & Phillips, T. G. Discovery of interstellar hydrogen fluoride. Astrophys. J. 488, L141–L144 (1997).
Agundez, M. et al. HIFI detection of hydrogen fluoride in the carbon star envelope IRC+10216. Astron. Astrophys. 533, L6 (2011).
Emprechtinger, M. et al. Hydrogen fluoride in high-mass star-forming regions. Astrophys. J. 756, 136 (2012).
Monje, R. R. et al. Herschel/HIFI observations of hydrogen fluoride toward Sagittarius B2(M). Astrophys. J. 734, L23 (2011).
Monje, R. R. et al. Discovery of hydrogen fluoride in the cloverleaf quasar at z = 2.56. Astrophys. J. 742, L21 (2011).
Neufeld, D. A. et al. Strong absorption by interstellar hydrogen fluoride: Herschel/HIFI observations of the sight-line to G10.6-0.4 (W31C). Astron. Astrophys. 518, L108 (2010).
Phillips, T. G. et al. Herschel observations of EXtra-Ordinary Sources (HEXOS): detection of hydrogen fluoride in absorption towards Orion KL. Astron. Astrophys. 518, L109 (2010).
Sonnentrucker, P. et al. Detection of hydrogen fluoride absorption in diffuse molecular clouds with Herschel/HIFI: an ubiquitous tracer of molecular gas. Astron. Astrophys. 521, L12 (2010).
van der Tak, F. The first results from the Herschel-HIFI mission. Adv. Space Res. 49, 1395–1407 (2012).
Zhu, C., Krems, R., Dalgarno, A. & Balakrishnan, N. Chemistry of hydrogen fluoride in the interstellar medium. Astrophys. J. 577, 795–797 (2002).
Neufeld, D. A., Wolfire, M. G. & Schilke, P. The chemistry of fluorine-bearing molecules in diffuse and dense interstellar gas clouds. Astrophys. J. 628, 260–274 (2005).
Wurzberg, E. & Houston, P. L. The temperature-dependence of absolute rate constants for the F + H2 and F + D2 reactions. J. Chem. Phys. 72, 4811–4814 (1980).
Stevens, P. S., Brune, W. H. & Anderson, J. G. Kinetic and mechanistic investigations of F + H2O/D2O and F + H2/D2 over the temperature-range 240–373 K. J. Phys. Chem. 93, 4068–4079 (1989).
Lique, F., Li, G. L., Werner, H. J. & Alexander, M. H. Communication: non-adiabatic coupling and resonances in the F + H2 reaction at low energies. J. Chem. Phys. 134, 231101 (2011).
Jaques, C. et al. Photoinitiated processes in complexes—subpicosecond studies of CO2-HI and stereospecificity in Ar-HX. J. Chem. Soc. Faraday Trans. 89, 1419–1425 (1993).
Persky, A. & Kornweitz, H. The kinetics of the reaction F + H2 → HF + H. A critical review of literature data. Int. J. Chem. Kinet. 29, 67–71 (1997).
Aquilanti, V. et al. Exact activation energies and phenomenological description of quantum tunneling for model potential energy surfaces. The F + H2 reaction at low temperature. Chem. Phys. 398, 186–191 (2012).
Neufeld, D. A. & Wolfire, M. G. The chemistry of interstellar molecules containing the halogen elements. Astrophys. J. 706, 1594–1604 (2009).
Godard, B. et al. Comparative study of CH+ and SH+ absorption lines observed towards distant star-forming regions. Astron. Astrophys. 540, A87 (2012).
Zhu, C., Krems, R., Dalgarno, A. & Balakrishnan, N. Erratum: ‘Chemistry of hydrogen fluoride in the interstellar medium’ (vol. 577, p. 795, 2002). Astrophys. J. 703, 1176 (2009).
Indriolo, N., Neufeld, D. A., Seifahrt, A. & Richter, M. J. Direct determination of the HF/H2 abundance ratio in interstellar gas. Astrophys. J. 764, 188 (2013).
Sims, I. R. et al. Ultralow temperature kinetics of neutral–neutral reactions—the technique and results for the reactions CN + O2 down to 13 K and CN + NH3 down to 25 K. J. Chem. Phys. 100, 4229–4241 (1994).
Nizkorodov, S. A., Harper, W. W. & Nesbitt, D. J. State-to-state reaction dynamics in crossed supersonic jets: threshold evidence for non-adiabatic channels in F + H2 . Faraday Discuss. 113, 107–117 (1999).
Werner, H. J., Kallay, M. & Gauss, J. The barrier height of the F + H2 reaction revisited: coupled-cluster and multireference configuration-interaction benchmark calculations. J. Chem. Phys. 128, 034305 (2008).
Skouteris, D., Castillo, J. F. & Manolopoulos, D. E. ABC: a quantum reactive scattering program. Comput. Phys. Commun. 133, 128–135 (2000).
Acknowledgements
The authors acknowledge support from the French Agence Nationale de Recherche (ANR Blanc Programme, Project CRNS) and the Centre National de la Recherche Scientifique (CNRS) via the Institut National des Sciences de l'Univers (INSU) Programme National de Physique et Chimie du Milieu Interstellaire. The authors thank D. Travers, J. Courbe, E. Gallou and J. Sorieux for technical support. M.T. thanks the French Ministère de l'Enseignement Supérieur et de la Recherche for a research studentship (Allocation Fléchée). S.D.L.P. acknowledges financial support from the Institut Universitaire de France. M.H.A. is grateful to the US National Science Foundation for support (grant CHE–1213332), to H-J. Werner and G. Li for their invaluable contributions in the construction of the LWAL FH2 PESs and to N. Brown for her help in estimating the F–H2 and H–H2 diffusion rate coefficients. The authors thank B. Godard for discussions regarding his astrochemical model.
Author information
Authors and Affiliations
Contributions
M.T., S.D.L.P., C.B. and I.R.S. carried out the experimental measurements and data analysis. A.C. designed the low-temperature hydrogen Laval nozzles. F.L. and M.H.A. performed the theoretical calculations. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 1483 kb)
Rights and permissions
About this article
Cite this article
Tizniti, M., Le Picard, S., Lique, F. et al. The rate of the F + H2 reaction at very low temperatures. Nature Chem 6, 141–145 (2014). https://doi.org/10.1038/nchem.1835
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1835
This article is cited by
-
Tunnelling measured in a very slow ion–molecule reaction
Nature (2023)
-
Understanding the Conformational Interconversions of a Polymer Chain in a Liquid Environment at the Single-molecule Level
Chemical Research in Chinese Universities (2023)
-
Renormalized chemical kinetics and benchmark quantum mechanical rates: activation energies and tunnelling transitivities for the reactions of fluorine atoms with H2 and HD
Rendiconti Lincei. Scienze Fisiche e Naturali (2023)
-
Intersystem crossing in the entrance channel of the reaction of O(3P) with pyridine
Nature Chemistry (2022)
-
Towards chemistry at absolute zero
Nature Reviews Chemistry (2021)