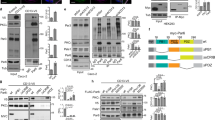
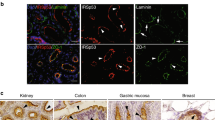
Abstract
To form epithelial organs cells must polarize and generate de novo an apical domain and lumen. Epithelial polarization is regulated by polarity complexes that are hypothesized to direct downstream events, such as polarized membrane traffic, although this interconnection is not well understood. We have found that Rab11a regulates apical traffic and lumen formation through the Rab guanine nucleotide exchange factor (GEF), Rabin8, and its target, Rab8a. Rab8a and Rab11a function through the exocyst to target Par3 to the apical surface, and control apical Cdc42 activation through the Cdc42 GEF, Tuba. These components assemble at a transient apical membrane initiation site to form the lumen. This Rab11a-directed network directs Cdc42-dependent apical exocytosis during lumen formation, revealing an interaction between the machineries of vesicular transport and polarization.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Bryant, D. M. & Mostov, K. E. From cells to organs: building polarized tissue. Nat. Rev. Mol. Cell Biol. 9, 887–901 (2008).
Stenmark, H. Rab GTPases as coordinators of vesicle traffic. Nat. Rev. Mol. Cell Biol. 10, 513–525 (2009).
He, B. & Guo, W. The exocyst complex in polarized exocytosis. Curr. Opin. Cell Biol. 21, 537–542 (2009).
Goldstein, B. & Macara, I. G. The PAR proteins: fundamental players in animal cell polarization. Dev. Cell. 13, 609–622 (2007).
Meder, D., Shevchenko, A., Simons, K. & Fullekrug, J. Gp135/podocalyxin and NHERF-2 participate in the formation of a preapical domain during polarization of MDCK cells. J. Cell Biol. 168, 303–313 (2005).
Martin-Belmonte, F. et al. PTEN-mediated apical segregation of phosphoinositides controls epithelial morphogenesis through Cdc42. Cell 128, 383–397 (2007).
Ferrari, A., Veligodskiy, A., Berge, U., Lucas, M. S. & Kroschewski, R. ROCK-mediated contractility, tight junctions and channels contribute to the conversion of a preapical patch into apical surface during isochoric lumen initiation. J. Cell Sci. 121, 3649–3663 (2008).
Casanova, J. E. et al. Association of Rab25 and Rab11a with the apical recycling system of polarized Madin-Darby canine kidney cells. Mol. Biol. Cell 10, 47–61 (1999).
Shaye, D. D., Casanova, J. & Llimargas, M. Modulation of intracellular trafficking regulates cell intercalation in the Drosophila trachea. Nat. Cell Biol. 10, 964–970 (2008).
Li, B. X., Satoh, A. K. & Ready, D. F. Myosin V, Rab11 and dRip11 direct apical secretion and cellular morphogenesis in developing Drosophila photoreceptors. J. Cell Biol. 177, 659–669 (2007).
Desclozeaux, M. et al. Active Rab11 and functional recycling endosome are required for E-cadherin trafficking and lumen formation during epithelial morphogenesis. Am. J. Physiol. Cell Physiol. 295, C545–556 (2008).
Knodler, A. et al. Coordination of Rab8 and Rab11 in primary ciliogenesis. Proc. Natl Acad. Sci. USA 107, 6346–6351 (2010).
Yoshimura, S., Egerer, J., Fuchs, E., Haas, A. K. & Barr, F. A. Functional dissection of Rab GTPases involved in primary cilium formation. J. Cell Biol. 178, 363–369 (2007).
Wu, S., Mehta, S. Q., Pichaud, F., Bellen, H. J. & Quiocho, F. A. Sec15 interacts with Rab11 via a novel domain and affects Rab11 localization in vivo. Nat. Struct. Mol. Biol. 12, 879–885 (2005).
Oztan, A. et al. Exocyst requirement for endocytic traffic directed toward the apical and basolateral poles of polarized MDCK cells. Mol. Biol. Cell 18, 3978–3992 (2007).
Zuo, X., Guo, W. & Lipschutz, J. H. The exocyst protein Sec10 is necessary for primary ciliogenesis and cystogenesis in vitro. Mol. Biol. Cell 20, 2522–2529 (2009).
Lalli, G. RalA and the exocyst complex influence neuronal polarity through PAR-3 and aPKC. J. Cell Sci. 122, 1499–1506 (2009).
Hayes, M. J. & Moss, S. E. Annexin 2 has a dual role as regulator and effector of v-Src in cell transformation. J. Biol. Chem. 284, 10202–10210 (2009).
Zobiack, N., Rescher, U., Ludwig, C., Zeuschner, D. & Gerke, V. The annexin 2/S100A10 complex controls the distribution of transferrin receptor-containing recycling endosomes. Mol. Biol. Cell 14, 4896–4908 (2003).
Rodriguez-Fraticelli, A. E. et al. The Cdc42 GEF intersectin 2 controls mitotic spindle orientation to form the lumen during epithelial morphogenesis. J. Cell Biol. 189, 725–738 (2010).
Qin, Y., Meisen, W. H., Hao, Y. & Macara, I. G. Tuba, a Cdc42 GEF, is required for polarized spindle orientation during epithelial cyst formation. J. Cell Biol. 189, 661–669 (2010).
Lubarsky, B. & Krasnow, M. A. Tube morphogenesis. Making and shaping biological tubes. Cell 112, 19–28 (2003).
Bagnat, M., Cheung, I. D., Mostov, K. E. & Stainier, D. Y. Genetic control of single lumen formation in the zebrafish gut. Nat. Cell Biol. 9, 954–960 (2007).
Ortiz, D., Medkova, M., Walch-Solimena, C. & Novick, P. Ypt32 recruits the Sec4p guanine nucleotide exchange factor, Sec2p, to secretory vesicles; evidence for a Rab cascade in yeast. J. Cell Biol. 157, 1005–1015 (2002).
Schluter, M. A. et al. Trafficking of Crumbs3 during cytokinesis is crucial for lumen formation. Mol. Biol. Cell 20, 4652–4663 (2009).
Morais-de-Sa, E., Mirouse, V. & St Johnston, D. aPKC phosphorylation of Bazooka defines the apical/lateral border in Drosophila epithelial cells. Cell 141, 509–523 (2010).
Walther, R. F. & Pichaud, F. Crumbs/DaPKC-dependent apical exclusion of Bazooka promotes photoreceptor polarity remodeling. Curr. Biol. 20, 1065–1074 (2010).
Joberty, G., Petersen, C., Gao, L. & Macara, I. G. The cell-polarity protein Par6 links Par3 and atypical protein kinase C to Cdc42. Nat. Cell Biol. 2, 531–539 (2000).
Jaffe, A. B., Kaji, N., Durgan, J. & Hall, A. Cdc42 controls spindle orientation to position the apical surface during epithelial morphogenesis. J. Cell. Biol. 183, 625–633 (2008).
Harris, K. P. & Tepass, U. Cdc42 and vesicle trafficking in polarized cells. Traffic 11, 1272–1279 (2010).
Balklava, Z., Pant, S., Fares, H. & Grant, B. D. Genome-wide analysis identifies a general requirement for polarity proteins in endocytic traffic. Nat. Cell Biol. 9, 1066–1073 (2007).
Shivas, J. M., Morrison, H. A., Bilder, D. & Skop, A. R. Polarity and endocytosis: reciprocal regulation. Trends Cell Biol. 20, 445–452 (2010).
Hogan, B. L. & Kolodziej, P. A. Organogenesis: molecular mechanisms of tubulogenesis. Nat. Rev. Genet. 3, 513–523 (2002).
Brignoni, M. et al. Exocytosis of vacuolar apical compartment (VAC) in Madin-Darby canine kidney epithelial cells: cAMP is involved as second messenger. Exp. Cell. Res. 205, 171–178 (1993).
Matheson, J., Yu, X., Fielding, A. B. & Gould, G. W. Membrane traffic in cytokinesis. Biochem. Soc. Trans. 33, 1290–1294 (2005).
Zhang, H., Squirrell, J. M. & White, J. G. RAB-11 permissively regulates spindle alignment by modulating metaphase microtubule dynamics in Caenorhabditis elegans early embryos. Mol. Biol. Cell 19, 2553–2565 (2008).
Pohl, C. & Jentsch, S. Final stages of cytokinesis and midbody ring formation are controlled by BRUCE. Cell 132, 832–845 (2008).
Horne-Badovinac, S. et al. Positional cloning of heart and soul reveals multiple roles for PKC lambda in zebrafish organogenesis. Curr. Biol. 11, 1492–1502 (2001).
Zheng, Z. et al. LGN regulates mitotic spindle orientation during epithelial morphogenesis. J. Cell Biol. 189, 275–288 (2010).
Yu, W. et al. Formation of cysts by alveolar type II cells in three-dimensional culture reveals a novel mechanism for epithelial morphogenesis. Mol. Biol. Cell 18, 1693–1700 (2007).
Liu, K. D. et al. Rac1 is required for reorientation of polarity and lumen formation through a PI 3-kinase-dependent pathway. Am. J. Physiol. Renal Physiol. 293, F1633–F1640 (2007).
Tanimizu, N., Miyajima, A. & Mostov, K. E. Liver progenitor cells fold up a cell monolayer into a double-layered structure during tubular morphogenesis. Mol. Biol. Cell. 20, 2486–2494 (2009).
Wang, A. Z., Wang, J. C., Ojakian, G. K. & Nelson, W. J. Determinants of apical membrane formation and distribution in multicellular epithelial MDCK cysts. Am. J. Physiol. 267, C473–C481 (1994).
Schuck, S., Manninen, A., Honsho, M., Fullekrug, J. & Simons, K. Generation of single and double knockdowns in polarized epithelial cells by retrovirus-mediated RNA interference. Proc. Natl Acad. Sci. USA 101, 4912–4917 (2004).
Moffat, J. et al. A lentiviral RNAi library for human and mouse genes applied to an arrayed viral high-content screen. Cell 124, 1283–1298 (2006).
Sfakianos, J. et al. Par3 functions in the biogenesis of the primary cilium in polarized epithelial cells. J. Cell Biol. 179, 1133–1140 (2007).
Ory, D. S., Neugeboren, B. A. & Mulligan, R. C. A stable human-derived packaging cell line for production of high titer retrovirus/vesicular stomatitis virus G pseudotypes. Proc. Natl Acad. Sci. USA 93, 11400–11406 (1996).
Hattula, K., Furuhjelm, J., Arffman, A. & Peranen, J. A Rab8-specific GDP/GTP exchange factor is involved in actin remodeling and polarized membrane transport. Mol. Biol. Cell 13, 3268–3280 (2002).
Kreitzer, G. et al. Three-dimensional analysis of post-Golgi carrier exocytosis in epithelial cells. Nat. Cell Biol. 5, 126–136 (2003).
Mangravite, L. M., Lipschutz, J. H., Mostov, K. E. & Giacomini, K. M. Localization of GFP-tagged concentrative nucleoside transporters in a renal polarized epithelial cell line. Am. J. Physiol. Renal. Physiol. 280, F879–F885 (2001).
Kreis, T. E. Microinjected antibodies against the cytoplasmic domain of vesicular stomatitis virus glycoprotein block its transport to the cell surface. Embo J. 5, 931–941 (1986).
Brown, P. S. et al. Definition of distinct compartments in polarized Madin-Darby canine kidney (MDCK) cells for membrane-volume sorting, polarized sorting and apical recycling. Traffic 1, 124–140 (2000).
Sato, Y. et al. Asymmetric coiled-coil structure with guanine nucleotide exchange activity. Structure 15, 245–252 (2007).
Acknowledgements
We thank F. Barr, E. Brown, J. Stow, W. Guo, I. Macara, K. Simons, J. Wilson, T. Weimbs, and A. Zahraoui for gifts of reagents and unpublished data, and the Mostov lab for kind assistance. Supported by a Susan G Komen Foundation Fellowship (D.M.B.), a DOD Breast Cancer Concept Award (A.D.), NIH grants R01DK074398, R01AI25144 and P01AI53194 (K.E.M.), grants of the Human Frontiers Science Program (HFSP-CDA00011/2009), Marie Curie (IRG-209382), MICINN (BFU2008-01916) and (CONSOLIDER CSD2009-00016) to F.M.-B.; and a JAE fellowship (MICINN) to A.E.R.F.
Author information
Authors and Affiliations
Contributions
D.M.B., A.D., A.R.F, F.M.B. and J.P. designed the experiments. D.M.B., A.D., A.R.F. and J.P. did the experimental work. D.M.B. and K.E.M. analysed the experiments. D.M.B. and K.E.M wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 2093 kb)
Rights and permissions
About this article
Cite this article
Bryant, D., Datta, A., Rodríguez-Fraticelli, A. et al. A molecular network for de novo generation of the apical surface and lumen. Nat Cell Biol 12, 1035–1045 (2010). https://doi.org/10.1038/ncb2106
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2106
This article is cited by
-
DYRK1-mediated phosphorylation of endocytic components is required for extracellular lumen expansion in ascidian notochord
Biological Research (2023)
-
PATJ inhibits histone deacetylase 7 to control tight junction formation and cell polarity
Cellular and Molecular Life Sciences (2023)
-
Apical–basal polarity and the control of epithelial form and function
Nature Reviews Molecular Cell Biology (2022)
-
Traject3d allows label-free identification of distinct co-occurring phenotypes within 3D culture by live imaging
Nature Communications (2022)
-
Two isoleucyl tRNAs that decode synonymous codons divergently regulate breast cancer metastatic growth by controlling translation of proliferation-regulating genes
Nature Cancer (2022)