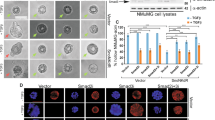
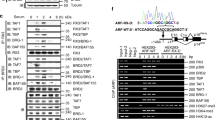
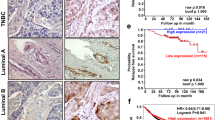
Abstract
Defining the functional modules within transcriptional regulatory factors that govern switching between repression and activation events is a central issue in biology. Recently, we have reported the dynamic role of a β-catenin–reptin chromatin remodelling complex in regulating a metastasis suppressor gene KAI1 (ref.1), which is capable of inhibiting the progression of tumour metastasis2,3,4,5. Here, we identify signalling factors that confer repressive function on reptin and hence repress the expression of KAI1. Biochemical purification of a reptin-containing complex has revealed the presence of specific desumoylating enzymes that reverse the sumoylation of reptin that underlies its function as a repressor. Desumoylation of reptin alters the repressive function of reptin and its association with HDAC1. Furthermore, the sumoylation status of reptin modulates the invasive activity of cancer cells with metastatic potential. These data clearly define a functional model and provide a novel link for SUMO modification in cancer metastasis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
16 June 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41556-021-00715-9
References
Kim, J. H. et al. Transcriptional regulation of a metastasis suppressor gene by Tip60 and β-catenin complexes. Nature 434, 921–926 (2005).
Shevde, L. A. & Welch, D. R. Metastasis suppressor pathways — an evolving paradigm. Cancer Lett. 198, 1–20 (2003).
Steeg, P. S. Metastasis suppressors alter the signal transduction of cancer cell. Nature Rev. Cancer 3, 55–63 (2002).
Petrylak, D. P. Metastases suppressors and prostate cancer. Nature Med. 1, 739–740 (1995).
Dong, J. -T. et al. KAI1, a metastasis suppressor gene for prostate cancer on human chromosome 11p11.2. Science 268, 884–886 (1995).
Glass, C. K. & Rosenfeld, M. G. The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev. 14, 121–141 (2000).
McKenna, N. J. & O'Malley, B. W. Combinatorial control of gene expression by nuclear receptors and coregulators. Cell 108, 465–474 (2002).
Baek, S. H. & Rosenfeld, M. G. Nuclear receptor coregulators: their modification codes and regulatory mechanism by translocation. Biochem. Biophys. Res. Commun. 319, 707–714 (2004).
Bauer, A. et al. Pontin52 and Reptin52 function as antagonistic regulators of β-catenin signaling activity. EMBO J. 19, 6121–6130 (2000).
Feng, Y., Lee, N. & Fearon, E. R. TIP49 regulates β-catenin-mediated neoplastic transformation and T-cell factor target gene induction via effects on chromatin remodeling. Cancer Res. 63, 8726–8734 (2003).
Kanemaki, M. et al. TIP49b, a new RuvB-like DNA helicase, is included in a complex together with another RuvB-like DNA helicase, TIP49a. J. Biol. Chem. 274, 22437–22444 (1999).
Jonsson, Z. O., Jha, S., Wohlschlegel J. A. & Dutta, A. Rvb1p/Rvb2p recruit Arp5p and assemble a functional Ino80 chromatin remodeling complex. Mol. Cell 16, 465–477 (2004).
Francis, N. J., Saurin, A. J., Shao, Z. & Kingston, R. E. Reconstitution of a functional core polycomb repressive complex. Mol. Cell 8, 545–556 (2001).
Ikura, T. et al. Involvement of the TIP60 histone acetylase complex in DNA repair and apoptosis. Cell 102, 463–473 (2000).
Fuchs, M. et al. The p400 complex is an essential E1A transformation target. Cell 106, 297–307 (2001).
Billin, A. N., Thirlwell, H. & Ayer, D. E. β-catenin-histone deacetylase interactions regulate the transition of LEF1 from a transcriptional repressor to an activator. Mol. Cell Biol. 20, 6882–6690 (2000).
Baek, S. H. et al. Regulated subset of G1 growth-control genes in response to derepression by the Wnt pathway. Proc. Natl. Acad. Sci. USA 100, 3245–3250 (2003).
Seeler, J. S. et al. Common properties of nuclear body protein SP100 and TIF1α chromatin factor: role of SUMO modification. Mol. Cell Biol. 21, 3314–3324 (2001).
Tatham, M. H. et al. Polymeric chains of SUMO-2 and SUMO-3 are conjugated to protein substrates by SAE1/SAE2 and Ubc9. J. Biol. Chem. 276, 35368–35374 (2001).
Johnson, E. S. Protein modification by SUMO. Annu. Rev. Biochem. 73, 355–382 (2004).
Li, S. J. & Hochstrasser, M. A new protease required for cell-cycle progression in yeast. Nature 398, 246–351 (1999).
Kim, K. I. et al. A new SUMO-1-specific protease, SUSP1, that is highly expressed in reproductive organs. J. Biol. Chem. 275, 14102–14106 (2000).
Best, J. L. et al. SUMO-1 protease-1 regulates gene transcription through PML. Mol. Cell 10, 843–855 (2002).
Bachant, J., Alcasabas, A., Blat, Y., Kleckner, N. & Elledge, S. J. The SUMO-1 isopeptidase Smt4 is linked to centromeric cohesion through SUMO-1 modification of DNA topoisomerase II. Mol. Cell 9, 1169–1182 (2002).
Tetsu, O. & McCormick, F. β-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 398, 422–426 (1999).
Huang, T. T., Wuerzberger-Davis, S. M., Wu, Z. H. & Miyamoto, S. Sequential modification of NEMO/IKKγ by SUMO-1 and ubiquitin mediates NF-κB activation by genotoxic stress. Cell 115, 565–576 (2003).
Ross, S., Best, J. L., Zon, L. I. & Gill, G. SUMO-1 modification represses Sp3 transcriptional activation and modulates its subnuclear localization. Mol. Cell 10, 831–842 (2002).
Salghetti, S. E., Caudy, A. A., Chenoweth, J. G. & Tansey, W. P. Regulation of transcriptional activation domain function by ubiquitin. Science 293, 1651–1653 (2001).
Alarcon-Vargas, D. & Ronai, Z. SUMO in cancer-wrestlers wanted. Cancer Biol. Therapy 1, 237–242 (2002).
Zheng, G. & Yang, Y. C. ZNF76, a novel transcriptional repressor targeting TATA-binding protein, is modulated by sumoylation. J. Biol. Chem. 279, 42410–42421 (2004).
Girdwood, D. et al. p300 transcriptional repression is mediated by SUMO modification. Mol. Cell 11, 1043–1054 (2003).
Baek, S. H. et al. Exchange of N-CoR corepressor and Tip60 coactivator complexes links gene expression by NF-κB and β-amyloid precursor protein. Cell 110, 55–67 (2002).
Cheng, J. et al. SENP1 enhances androgen receptor-dependent transcription through desumoylation of histone deacetylase 1. Mol. Cell Biol. 24, 6021–6028 (2004).
Kurihara, I. et al. Ubc9 and protein inhibitor of activated STAT1 activate chicken ovalbumin upstream promoter-transcription factor I-mediated human CYP11B2 gene transcription. J. Biol. Chem. 280, 6721–6730 (2005).
Elbashir, S. M. et al. Duplexes of 21-nucleotide RNAs mediate RNA interference in mammalian cell culture. Nature 411, 494–498 (2001).
Acknowledgements
We thank M. G. Rosenfeld (Howard Hughes Medical Institute), C. K. Glass (University of California at San Diego), C. Y. Choi (Sung Kyun Kwan University) and E. Park (Seoul National University) for critical reading of our manuscript. This work was supported by Korea Research Foundation Grant (MOEHRD, Basic Research Promotion Fund), the National Research and Development programme for cancer control from the Ministry of Health & Welfare, and Science Research Center (SRC) programme of the Ministry of Science and Technology (MOST) and the Koreas Science and Engineering Foundation (KOSEF), Brain Koreas 21 (BK21) and Seoul Science Fellowship (H.J.C and B.K), and Ubiquitome Research Program from MOST/KOSEF (C.H.C and K.I.K.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures S1, S2, S3 and S4 (PDF 86 kb)
Rights and permissions
About this article
Cite this article
Kim, J., Choi, H., Kim, B. et al. Roles of sumoylation of a reptin chromatin-remodelling complex in cancer metastasis. Nat Cell Biol 8, 631–639 (2006). https://doi.org/10.1038/ncb1415
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1415
This article is cited by
-
Protein post-translational modifications in the regulation of cancer hallmarks
Cancer Gene Therapy (2023)
-
SUMOylation inhibition enhances multiple myeloma sensitivity to lenalidomide
Cancer Gene Therapy (2023)
-
SUMOylation inhibition enhances dexamethasone sensitivity in multiple myeloma
Journal of Experimental & Clinical Cancer Research (2022)
-
X-ray structure of full-length human RuvB-Like 2 – mechanistic insights into coupling between ATP binding and mechanical action
Scientific Reports (2018)
-
SUMO and the robustness of cancer
Nature Reviews Cancer (2017)