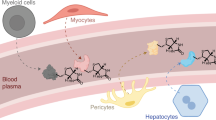
Abstract
A coordinated functional genomics program was implemented to identify secreted polypeptides with therapeutic applications in the treatment of diabetes. Secreted factors were predicted from a diverse expressed-sequence tags (EST) database, representing >1,000 cDNA libraries, using a combination of bioinformatic algorithms. Subsequently, ∼8,000 human proteins were screened in high-throughput cell-based assays designed to monitor key physiological transitions known to be centrally involved in the physiology of type 2 diabetes. Bone morphogenetic protein-9 (BMP-9) gave a positive response in two independent assays: reducing phosphoenolpyruvate carboxykinase (PEPCK) expression in hepatocytes and activating Akt kinase in differentiated myotubes. Purified recombinant BMP-9 potently inhibited hepatic glucose production and activated expression of key enzymes of lipid metabolism. In freely fed diabetic mice, a single subcutaneous injection of BMP-9 reduced glycemia to near-normal levels, with maximal reduction observed 30 hours after treatment. BMP-9 represents the first hepatic factor shown to regulate blood glucose concentration. Using a combination of bioinformatic and high-throughput functional analyses, we have identified a factor that may be exploited for the treatment of diabetes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Harris, M.I. et al. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The Third National Health and Nutrition Examination Survey, 1988–1994. Diabetes Care 21, 518–524 (1998).
Taylor, S.I. Deconstructing type 2 diabetes. Cell 97, 9–12 (1999).
Saltiel, A.R. New perspectives into the molecular pathogenesis and treatment of type 2 diabetes. Cell 104, 517–529 (2001).
Nordlie, R.C., Foster, J.D. & Lange, A.J. Regulation of glucose production by the liver. Annu. Rev. Nutr. 19, 379–406 (1999).
Czech, M.P. & Corvera, S. Signaling mechanisms that regulate glucose transport. J. Biol. Chem. 274, 1865–1868 (1999).
Bergman, R.N. & Ader, M. Free fatty acids and pathogenesis of type 2 diabetes mellitus. Trends Endocrinol. Metab. 11, 351–356 (2000).
Rorsman, P. The pancreatic β-cell as a fuel sensor: an electrophysiologist's viewpoint. Diabetologia 40, 487–495 (1997).
Adams, M.D. et al. Complementary DNA sequencing: expressed sequence tags and human genome project. Science 252, 1651–1656 (1991).
Boguski, M.S. & Schuler, G.D. ESTablishing a human transcript map. Nat. Genet. 10, 369–371 (1995).
Lander, E.S. et al. Initial sequencing and analysis of the human genome. Nature 409, 860–921 (2001).
Venter, J.C. et al. The sequence of the human genome. Science 291, 1304–1351 (2001).
Hogan, B.L. Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes Dev. 10, 1580–1594 (1996).
Eddy, S.R. Profile hidden Markov models. Bioinformatics 14, 755–763 (1998).
Barash, S., Wang, W. & Shi, Y. Human secretory signal peptide description by hidden Markov model and generation of a strong artificial signal peptide for secreted protein expression. Biochem. Biophys. Res. Commun. 294, 835–842 (2002).
Nielsen, H., Engelbrecht, J., Brunak, S. & von Heijne, G. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 10, 1–6 (1997).
Bairoch, A. & Boeckmann, B. The SWISS-PROT protein sequence data bank: current status. Nucleic Acids Res. 22, 3578–3580 (1994).
Magnusson, I., Rothman, D.L., Katz, L.D., Shulman, R.G. & Shulman, G.I. Increased rate of gluconeogenesis in type II diabetes mellitus. A 13C nuclear magnetic resonance study. J. Clin. Invest. 90, 1323–1327 (1992).
Cho, H. et al. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB-β). Science 292, 1728–1731 (2001).
Cross, D.A., Alessi, D.R., Cohen, P., Andjelkovich, M. & Hemmings, B.A. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378, 785–789 (1995).
Kraegen, E.W., Cooney, G.J., Ye, J.M., Thompson, A.L. & Furler, S.M. The role of lipids in the pathogenesis of muscle insulin resistance and β-cell failure in type II diabetes and obesity. Exp. Clin. Endocrinol. Diabetes 109, S189–S201 (2001).
Laakso, M. Insulin resistance and its impact on the approach to therapy of type 2 diabetes. Int. J. Clin. Pract. (Suppl.) 8–12 (2001).
Castelein, H. et al. The peroxisome proliferator activated receptor regulates malic enzyme gene expression. J. Biol. Chem. 269, 26754–26758 (1994).
Petty, K.J., Desvergne, B., Mitsuhashi, T. & Nikodem, V.M. Identification of a thyroid hormone response element in the malic enzyme gene. J. Biol. Chem. 265, 7395–7400 (1990).
Barroso, I. & Santisteban, P. Insulin-induced early growth response gene (Egr-1) mediates a short term repression of rat malic enzyme gene transcription. J. Biol. Chem. 274, 17997–18004 (1999).
Horton, J.D., Goldstein, J.L. & Brown, M.S. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Invest. 109, 1125–1131 (2002).
Becard, D. et al. Adenovirus-mediated overexpression of sterol regulatory element binding protein-1c mimics insulin effects on hepatic gene expression and glucose homeostasis in diabetic mice. Diabetes 50, 2425–2430 (2001).
Formisano, P. et al. Insulin-activated protein kinase Cβ bypasses Ras and stimulates mitogen-activated protein kinase activity and cell proliferation in muscle cells. Mol. Cell Biol. 20, 6323–6333 (2000).
Machinal-Quelin, F., Dieudonne, M.N., Leneveu, M.C., Pecquery, R. & Giudicelli, Y. Pro-adipogenic effect of leptin on rat pre-adipocytes in vitro: activation of MAPK and STAT3 signaling pathways. Am. J. Physiol. Cell Physiol. 282, C853–C863 (2002).
Blagoev, B. et al. Inhibition of adipocyte differentiation by resistin-like molecule? Biochemical characterization of its oligomeric nature. J. Biol. Chem. 277, 42011–42016 (2002).
Luo, G. et al. BMP-7 is an inducer of nephrogenesis, and is also required for eye development and skeletal patterning. Genes Dev. 9, 2808–2820 (1995).
Dong, J. et al. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature 383, 531–535 (1996).
Song, J.J. et al. Bone morphogenetic protein-9 binds to liver cells and stimulates proliferation. Endocrinology 136, 4293–4297 (1995).
Miller, A.F., Harvey, S.A., Thies, R.S. & Olson, M.S. Bone morphogenetic protein-9. An autocrine/paracrine cytokine in the liver. J. Biol. Chem. 275, 17937–17945 (2000).
Helm, G.A. et al. Use of bone morphogenetic protein-9 gene therapy to induce spinal arthrodesis in the rodent. J. Neurosurg. 92, 191–196 (2000).
Majumdar, M.K., Wang, E. & Morris, E.A. BMP-2 and BMP-9 promotes chondrogenic differentiation of human multipotential mesenchymal cells and overcomes the inhibitory effect of IL-1. J. Cell Physiol. 189, 275–284 (2001).
Varady, P. et al. Morphologic analysis of BMP-9 gene therapy–induced osteogenesis. Hum. Gene Ther. 12, 697–710 (2001).
Ploemacher, R.E., Engels, L.J., Mayer, A.E., Thies, S. & Neben, S. Bone morphogenetic protein 9 is a potent synergistic factor for murine hemopoietic progenitor cell generation and colony formation in serum-free cultures. Leukemia 13, 428–437 (1999).
Lopez-Coviella, I., Berse, B., Krauss, R., Thies, R.S. & Blusztajn, J.K. Induction and maintenance of the neuronal cholinergic phenotype in the central nervous system by BMP-9. Science 289, 313–316 (2000).
Lang, S., Goldstein, M.S. & Levine, R. Influence of the liver on uptake of glucose by peripheral tissues. Am. J. Physiol. 177, 447–450 (1955).
Mertz, W. & Schwartz, K. An effect of liver extracts on glucose tolerance in rats. Am. J. Physiol. 203, 533–556 (1962).
Lautt, W.W. The HISS story overview: a novel hepatic neurohumoral regulation of peripheral insulin sensitivity in health and diabetes. Can. J. Physiol. Pharmacol. 77, 553–562 (1999).
Fiscella, M. et al. TIP, a T cell factor identified using high-throughput screening approaches increases survival in an acute GVHD model. Nat. Biotechnol. 21, 302–307 (2003).
Boguski, M.S., Lowe, T.M. & Tolstoshev, C.M. dbEST—database for “expressed sequence tags”. Nat. Genet. 4, 332–333 (1993).
Ashburner, M. et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 25, 25–29 (2000).
Pruitt, K.D. & Maglott, D.R. RefSeq and LocusLink: NCBI gene-centered resources. Nucleic Acids Res. 29, 137–140 (2001).
Forrer, P., Tamaskovic, R. & Jaussi, R. Enzyme-linked immunosorbent assay for measurement of JNK, ERK, and p38 kinase activities. Biol. Chem. 379, 1101–1111 (1998).
Wang, J.C., Stafford, J.M., Scott, D.K., Sutherland, C. & Granner, D.K. The molecular physiology of hepatic nuclear factor 3 in the regulation of gluconeogenesis. J. Biol. Chem. 275, 14717–14721 (2000).
National Research Council. Guide for the Care and Use of Laboratory Animals (National Academy Press, Washington, DC, 1996).
Acknowledgements
We thank Viktor Roschke, Partha Chowdhury, Michael Bloom, and Kathy McCormick for technical assistance, and Paul Moore for a critical review of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Chen, C., Grzegorzewski, K., Barash, S. et al. An integrated functional genomics screening program reveals a role for BMP-9 in glucose homeostasis. Nat Biotechnol 21, 294–301 (2003). https://doi.org/10.1038/nbt795
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt795
This article is cited by
-
Effects of exogenous nerve growth factor on the expression of BMP-9 and VEGF in the healing of rabbit mandible fracture with local nerve injury
Journal of Orthopaedic Surgery and Research (2021)
-
New insights into BMP9 signaling in liver diseases
Molecular and Cellular Biochemistry (2021)
-
Potential roles of bone morphogenetic protein-9 in glucose and lipid homeostasis
Journal of Physiology and Biochemistry (2020)
-
A novel role of bone morphogenetic protein 6 (BMP6) in glucose homeostasis
Acta Diabetologica (2019)
-
BMP9-induced osteoblastic differentiation requires functional Notch signaling in mesenchymal stem cells
Laboratory Investigation (2019)