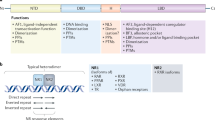
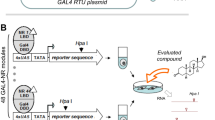
Abstract
There are two basic types of receptor transducing systems: those which utilize membrane bound receptors and are activated at the cell surface by the appropriate hormone and transmit their signal to the internae of the cell via a second messenger (i.e. cAMP), and those that utilize internal, cytoplasmic or nuclear receptors (intracellular receptors) which upon activation by hormones interact directly with DNA and alter the genetic program of a cell. This review focuses on the mechanism of action of these intracellular receptors and discusses how such an understanding is expected to facilitate the discovery of new therapeutic agents.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Evans, R.M. 1988. The steroid and thyroid hormone receptor superfamily. Science 240: 889–895.
Beato, M. 1989. Gene regulation by steroid hormones. Cell 56: 335–344.
O'Malley, B.W. and Tsai, M.J. 1992. Molecular pathways of steroid receptor action. Biol. Reprod. 46: 163–167.
Sunderland, M.C. and Osborne, C.K. 1991. Tamoxifen in pre-menopausal patients with rnetastatic breast cancer: a review. J. Clin. Oncol. 9: 1283–1297.
Rao, B.R. and Slotman, B.J. 1991. Endocrine factors in common epithelial ovarian cancer. Endocr. Rev. 12: 14–26.
Dreicer, R. and Wilding, G. 1992. Steroid hormone agonists and antagonists in the treatment of cancer. Cancer Investigation 10: 27–41.
Daneshgari, F. and Crawford, E.D. 1993. Endocrine therapy of advanced carcinoma of the prostate. Cancer 71: 1089–1097.
Barzel, U.S. 1988 Estrogens in the prevention and treatment of postmenopausal osteoporosis: a review. Am. J. Med. 85: 847–850.
Jordan, V.C., Fritz, N.F. and Tormey, D.C. 1987. Endocrine effects of adjuvant chemotherapy and long-term tamoxifen administration on node-positive patients with breast cancer. Cancer Res. 47: 624–630.
Henderson, B.E., Ross, R.K. and Pike, M.C. 1993. Hormonal chemoprevention of cancer in women. Science 259: 633–638.
Poisson, M., Pertuiset, B.F., Hauw, J.J., Philippon, J., Buge, A., Moguilewsky, M. and Philibert, D. 1983. Steroid hormone receptors in human meningiomas, gliomas and brain metastases. J. Neuro. One. 1: 179–189.
Kettel, L.M., Murphy, A.A., Mortola, J.F., Liu, J.H., Ulmann, A. and Yen, S.S. 1991. Endocrine responses to long-term administration of the anti-progesterone RU486 in patients with pelvic endometriosis. Fert. and Ster. 56: 402–407.
Baulieu, E.E. 1989. Contragestation and other clinical applications of RU486, an antiprogesterone at the receptor. Science 245: 1351–1357.
De Voogt, H.J. 1992. The position of cyproterone acetate (CPA), a steroidal anti-androgen, in the treatment of prostate cancer. Prostate 4: 91–95.
Benson, R.C. 1992. A rationale for the use of non-steroidal anti-androgens in the management of prostate cancer. Prostate 4: 85–90.
McDonnell, D.P., Mangelsdorf, D.J., Pike, J.W., Haussler, M.R. and O'Malley, B.W. 1987. Molecular cloning of complementary DNA encoding the avian receptor for vitamin D. Science 235: 1214–1217.
Fuller, P.J. 1991. The steroid receptor superfamily: mechanisms of diversity. FASEB J. 5: 3092–3099.
Dobson, A.D.W., Conneely, O.M., Beattie, W.G., Maxwell, B.L., Mak, P., Tsai, M.-J., Schrader, W.T. and O'Malley, B.W. 1989. Mutational analysis of the chicken progesterone receptor. J. Biol. Chem. 264: 4207–4211.
Tasset, D., Tora, L., Fromental, C., Scheer, E. and Chambon, P. 1990. Distinct classes of transcriptional activating domains function by different mechanisms. Cell 62: 1177–1187.
Metzger, D., Losson, R., Bornert, J.M., Lemoine, Y. and Chambon, P. 1992. Promoter specificity of the two transcriptional activation functions of the human oestrogen receptor in yeast. Nucleic Acids Res. 20: 2813–2817.
Jenster, G., van der Korput, H.A.G.M., Vroonhoven, C.V., van der Kwast, T.H., Trapman, J. and Brinkmann, A.O. 1991. Domains of the human androgen receptor involved in steroid binding, transcriptional activation, and subcellular localization. Mol. Endo. 5: 1396–1404.
Laudet, V., Hanni, C., Coll, F.C., Catzeflis, F. and Stehelin, D. 1992. Evolution of the nuclear receptor gene superfamily. EMBO J. 11: 1003–1013.
O'Malley, B.W. 1990. The steroid receptor superfamily: more excitement predicted for the future. Mol. Endo. 4: 363–369.
Mangelsdorf, D.J., Ong, E.S., Dyck, J.A. and Evans, R.M. 1990. Nuclear receptor that identifies a novel retinoic acid response pathway. Nature 345: 224–229.
Heyman, R., Mangelsdorf, D.J., Dyck, J.A., Stein, R.B., Eichele, G., Evans, R.M. and Thaller, C. 1992. 9-cis retinoic acid is a high affinity ligand for the retinoid X receptor. Cell 68: 397–406.
Levin, A.A., Sturzenbecker, L.J., Kazmer, S., Bosakowski, T., Huselton, C., Allenby, G., Speck, J., Kratzasen, C., Rosenberger, M. and Lovey, A. 1992. 9-Cis retinoic acid stereoisomer binds and activates the nuclear receptor RXR alpha. Nature 355: 359–361.
Issemann, I. and Green, S. 1990. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature 347: 645–650.
Keller, H., Dreyer, C., Medin, J., Mahfoudi, A., Ozato, K. and Wahli, W. 1993. Fatty acids and retinoids control lipid metabolism through activation of peroxisome proliferator-activated receptor-retinoid X receptor heterodimers. Proc. Natl. Acad. Sci. USA 90: 2160–2164.
Sher, T., Yi, H.F., McBride, O.W. and Gonzalez, F.J. 1993. cDNA cloning, chromosomal mapping, and functional characterization of the human peroxisome proliferator activated receptor. Biochemistry 32: 5598–5604.
Smith, D.F., and Toft, D.O. 1993. Steroid receptors and their associated proteins. Mol. Endo. 7: 4–11.
Picard, D., Kumar, V., Chambon, P. and Yamamoto, K.R. 1992. Signal transduction by steroid hormones: nuclear localization is differentially regulated in estrogen and glucocorticoid receptors. Cell Regul. 1: 291–299.
Sanchez, E.R., Hirst, M., Scherrer, L.C., Tang, H.Y., Welsh, M.J., Harmon, J.M., Simmons, S.S.J., Ringold, G.M. and Pratt, W.B. 1990. Hormone-free mouse glucocorticoid receptors overexpressed in Chinese hamster ovary cells are localized to the nucleus and are associated with both hsp 70 and hsp 90. J. Biol. Chem. 265: 20123–20130.
Allan, G.F., Leng, X., Tsai, S.Y., Weigel, N.L., Edwards, D.P., Tsai, M.J. and O'Malley, B.W. 1992. Hormone and antihormone induce distinct conformational changes which are central to steroid receptor activation. J. Biol. Chem. 267: 19513–19520.
Allan, G.F., Tsai, S.Y., Tsai, M.J. and O'Malley, B.W. 1992. Ligand-dependent conformational changes in the progesterone receptor are necessary for events that follow DNA binding. Proc. Natl. Acad. Sci. USA 89: 11750–11754.
Vegeto, E., Allan, G.F., Schrader, W.T., Tsai, M.J., McDonnell, D.P. and O'Malley, B.W. 1992. Mechanism of RU486 antagonism is dependent on the conformation of the carboxy-terminal tail of the human progesterone receptor. Cell 69: 703–713.
Lazar, M.A. and Chin, W.W. 1990. Nuclear thyroid hormone receptors. J. Clin. Invest. 86: 1777–1782.
Dalman, F.C., Sturzenbecker, L.J., Levin, A.A., Lucas, D.A., Perdew, G.H., Petkovitch, M., Chambon, P., Grippo, J.F. and Pratt, W.B. 1991. Retinoic acid receptor belongs to a subclass of nuclear receptors that do not form “docking” complexes with hsp90. Biochemistry 30: 5605–5608.
Pike, J.W. 1982. Interaction between 1,25-dihydroxyvitamin D3 receptors and intestinal nuclei. J. Biol. Chem. 257: 6766–6775.
Glass, C.K., Holloway, J.M., Devary, O.V. and Rosenfeld, M.G. 1988. The thyroid hormone receptor binds with opposite transcriptional effects to a common sequence motif in thyroid hormone and estrogen response elements. Cell 54: 313–323.
Tsai, S.Y., Carlstedt-Duke, J., Weigel, N.L., Dahlman, K., Gustafsson, J.A., Tsai, M.J. and O'Malley, B.W. 1988. Molecular interactions of steroid hormone receptor with its enhancer element: evidence for receptor dimer formation. Cell 55: 361–369.
Kliewer, S.A., Umesono, K., Mangelsdorf, D.J. and Evans, R.M. 1992. Retinoid X receptor interacts with nuclear receptors in retinoic acid, thyroid hormone and vitamin D3 signalling. Nature 355: 446–449.
Kliewer, S.A., Umesono, K., Mangelsdorf, D.J., Noonan, D., Heyman, R.A. and Evans, R.M. 1992. Convergence of 9-cis retinoic acid and peroxisome proliferator signalling pathways through heterodimer formation of their receptors. Nature 358: 771–774.
Umesono, K. and Evans, R.M. 1989. Determinants of target gene specificity for steroid/thyroid hormone receptors. Cell 57: 1139–1146.
Schule, R. and Evans, R.M. 1991. Cross-coupling of signal transduction pathways: zinc finger meets leucine zipper. Trends. Genet. 7: 377–381.
Power, R.F., Conneely, O.M. and O'Malley, B.W. 1992. New insights into activation of the steroid hormone receptor superfamily. Trends. Pharmacol. Sci. 13: 318–323.
Power, R.F., Lydon, J.P., Conneely, O.M. and O'Malley, B.W. 1991. Dopamine activation of an “orphan“ (COUP-TF) of the steroid receptor super-family. Science 252: 1546–1548.
Power, R.F., Mani, S.D., Codina, J., Conneely, O.M. and O'Malley, B.W. 1991. Dopaminergic and ligand-independent activation of steroid hormone receptors. Science 254: 1636–1639.
Lydon, J.P., Power, R.F. and Conneely, O.M. 1992. Differential modes of activation define orphan subclasses with the steroid/thyroid receptor superfamily. Gene Expr. 2: 273–283.
Smith, C.L., Conneely, O.M. and O'Malley, B.W. 1993 Modulation of the ligand-independent activation of the human estrogen receptor by hormone and antihormone. Proc. Natl. Acad. Sci. USA. In press
Denner, L.A., Weigel, N.L., Schrader, W.T. and O'Malley, B.W. 1989. Hormone-dependent regulation of chicken progesterone receptor deoxyribonucleic acid binding and phosphorylation. Endocrinology 125: 3051–3058.
Ali, S., Metzger, D., Bornert, J.M. and Chambon, P. 1993. Modulation of transcriptional activation by ligand-dependent phosphorylation of the human oestrogen receptor A/B region. EMBO J. 12: 1153–1160.
Pike, J.W. and Sleator, N.M. 1985. Hormone-dependent phosphorylation of the 1,25-dihydroxyvitamin D3 receptor in mouse fibroblasts. Biochem. Biophys. Res.Commun. 131: 378–385.
Denner, L.A., Weigel, N.L., Maxwell, B.L., Schrader, W.T. and O'Malley, B.W. 1990. Regulation of progesterone receptor-mediated transcription by phosphorylation. Science 250: 1740–1743.
Liu, J., Farmer, J.D., Jr., Lane, W.S., Friedman, J., Weismann, I. and Schreiber, S.L. 1991. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell 66: 807–815.
Ignar-Trowbridge, D.M., Teng, C.T., Ross, K.A., Parker, M.G., Korach, K.S. and McLachlan, J.A. 1993. Peptide growth factors elicit estrogen receptor-dependent transcriptional activation of an estrogen-responsive element. Mol. Endo. In press
Aronica, S.M. and Katzenellenbogen, B.S. 1993. Stimulation of estrogen receptor-mediated transcription and alteration in the phosphorylation state of the rat uterine estrogen receptor by estrogen, cyclic adenosine monophosphate, and insulin-like growth factor-1. Mol. Endo 7: 743–752.
Ignar-Trowbridge, D.M., Nelson, K.G., Bidwell, M.C., Curtis, S.W., Washbum, T.F., McLachlan, J.A. and Korach, K.S. 1992. Coupling of dual signaling pathways: Epidermal growth factor action involves the estrogen receptor. Proc. Natl. Acad. Sci. USA 89: 4658–4662.
Berry, M., Metzger, D. and Chambon, P. 1990. Role of the two activating domains of the estrogen receptor in the cell-type and promoter-context dependent agonist activity of the anti-estrogen 4-hydroxytamoxifen. EMBO J. 9: 2811–2818.
Klein-Hitpass, L., Cato, A.C.B., Henderson, D. and Ryffel, U.G. 1991. Two types of antiprogestins indentified by their differential action in transcriptionally active extracts from T47D cells. Nucl. Acids Res. 19: 1227–1233.
Bagchi, M.K., Elliston, J.F., Tsai, S.Y., Edwards, D.P., Tsai, M.J. and O'Malley, B.W. 1988. Steroid hormone dependent interaction of human progesterone receptor with its target enhancer element. Mol. Endo. 2: 1221–1229.
McDonnell, D.P., Nawaz, Z. and O'Malley, B.W. 1991. In situ distinction between steroid receptor binding and transactivation at a target gene. Mol. Cell Biol. 11: 4350–4355.
McDonnell, D.P., Vegeto, E. and O'Malley, B.W. 1992. Identification of a negative regulatory function for steroid receptors. Proc. Natl. Acad. Sci. USA 89: 10563–10567.
Keleher, C.A., Redd, M.J., Schultz, J., Carlson, M. and Johnson, A.D. 1992. Ssn6-Tup1 is a general represser of transcription in yeast. Cell 68: 709–719.
Williams, F.E. and Trembly, R.J. 1990. Characterization of TUP1, a mediator of glucose repression in Saccharomyces cerevisiae. Mol. Cell Biol. 10: 6500–6511.
Pham, T.A., Hwung, Y-R., Santiso-Mere, D., McDonnell, D.P. and O'Malley, B.W. 1992. Ligand-dependent and independent function of the transactivation regions of the human estrogen receptor in yeast. Mol. Endo. 6: 1043–1050.
Tora, L., White, J., Brou, C., Tasset, D., Webster, N., Scheer, E. and Chambon, P. 1989. The human estrogen receptor has two independent nonacidic transcriptional activation functions. Cell 59: 477–487.
Berger, T.S., Parandoosh, Z., Perry, B.W. and Stein, R.B. 1992. Interaction of glucocorticoid analogues with the human glucocorticoid receptor. J. Steroid. Biochem. Mol. Biol. 41: 733–738.
Wakeling, A.E., Dukes, M. and Bowler, J. 1991. A potent specific pure antiestrogen with clinical potential. Cancer Res. 51: 3867–3873.
Elliston, J.F., Tsai, S.Y., O'Malley, B.W. and Tsai, M.J. 1990. Superactive estrogen receptors. Potent activators of gene expression. J. Biol. Chem. 265: 11517–11521.
Pham, T.A., Elliston, J.F., Nawaz, Z., McDonnell, D.P., Tsai, M.J. and O'Malley, B.W. 1991. Anti-estrogen can establish nonproductive receptor complexes and alter chromatin structure at target enhancers. Proc. Natl. Acad. Sci. USA 88: 3125–3129.
Kakizuka, A., Miller, W.H.J., Umesono, K., Warrell, R.P.J., Frankel, S.R., Murty, V.V., Dmitrovsky, E. and Evans, R.M. 1991. Chromosomal translocation t (15;17) in human acute promyelocytic leukemia fuses RAR alpha with a novel putative transcription factor, PML. Cell 66: 663–674.
Allegretto, E.A., McClurg, M.R., Lazarchik, S.B., Clemm, D.L., Kerner, S.A., Elgort, M.G., Boehm, M.F., White, S.K., Pike, J.W. and Heyman, R.A. 1993. Transactivation properties of retinoic acid and retinoid X receptors in mammalian cells and yeast: correlation with hormone binding and effects of metabolism. J. Biol. Chem. In press.
Lazar, M.A. 1991. Steroid and thyroid hormone receptors. Endocrinol. Metab. Clin. Am. 20: 681–695.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
McDonnell, D., Vegeto, E. & Gleeson, M. Nuclear Hormone Receptors as Targets for New Drug Discovery. Nat Biotechnol 11, 1256–1261 (1993). https://doi.org/10.1038/nbt1193-1256
Issue Date:
DOI: https://doi.org/10.1038/nbt1193-1256
This article is cited by
-
Diet, fatty acids, and regulation of genes important for heart disease
Current Atherosclerosis Reports (2004)