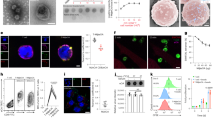
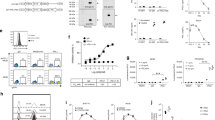
Abstract
High-level systemic delivery of viral vectors to tumors has proved problematic as a result of immune neutralization, nonspecific adhesion, and clearance of circulating viral particles. Some cell types localize to tumors in response to particular biological properties associated with tumor growth. Their use to deliver viral vectors to tumors would allow precious viral stocks to be protected until they can be released at high local concentrations. Here, we describe a mechanism by which retroviral vector production by T cells can be regulated by a tumor-specific trigger through engagement of a chimeric immune receptor (CIR) with its target antigen. The virus that is released from the T cells can also be transcriptionally targeted. Finally, we show that it is possible to use vector-loaded, antigen-triggered human T cells as therapeutic, tumor-specific vector delivery cells in models of both local intratumoral and systemic delivery to both lung and liver metastases. This strategy incorporates multiple levels of targeting into the delivery system at the stages of surface targeting, viral production, and gene expression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Verma, I. & Somia, N. Gene therapy—promises, problems and prospects. Nature 389, 239–242 (1997).
Vile, R.G., Russell, S.J. & Lemoine, N.R. Cancer gene therapy: hard lessons and new courses. Gene Ther. 7, 2–8 (2000).
Russell, S.J. & Cosset, F.-J. Modifying the host range properties of retroviral vectors. J. Gene Med. 1, 300–311 (1999).
Grill, J. et al. Combined targeting of adenoviruses to integrins and epidermal growth factor receptors increases gene transfer into primary glioma cells and spheroids. Clin. Cancer Res. 7, 641–650 (2001).
Krasnykh, V., Belousova, N., Korokhov, N., Mikheeva, G. & Curiel, D.T. Genetic Targeting of an adenovirus vector via replacement of the fiber protein with the phage T4 fibritin. J. Virol. 75, 4176–4183 (2001).
Wu, P. et al. Mutational analysis of the adeno-associated virus type 2 (AAV2) capsid gene and construction of AAV2 vectors with altered tropism. J. Virol. 74, 8635–8647 (2000).
Kirn, D., Martuza, R.L. & Zwiebel, J. Replication-selective virotherapy for cancer: biological principles, risk management and future directions. Nat. Med. 7, 781–787 (2001).
Alemany, R., Balague, C. & Curiel, D.T. Replicative adenoviruses for cancer therapy. Nat. Biotechnol. 18, 723–727 (2000).
Takeuchi, Y. et al. Sensitization of cells and retroviruses to human serum by (α1-3) galactosyltransferase. Nature 379, 85–88 (1996).
Chirmule, N. et al. Readministration of adenovirus vector in nonhuman primate lungs by blockade of CD40–CD40 ligand interactions. J. Virol. 74, 3345–3352 (2000).
Paillard, F. Circumventing adenovirus immune response to achieve long-term correction of genetic diseases. Hum. Gene Ther. 9, 454–456 (1998).
Pizzato, M., Marlow, S.A., Blair, E.D. & Takeuchi, Y. Initial binding of murine leukemia virus particles to cells does not require specific Env-receptor interaction. J. Virol. 73, 8599–8611 (1999).
Alemany, R., Suzuki, K. & Curiel, D.T. Blood clearance rates of adenovirus type 5 in mice. J. Gen. Virol. 81(Pt 11), 2605–2609 (2000).
Griffiths, L. et al. The macrophage—a novel system to deliver gene therapy to pathological hypoxia. Gene Ther. 7, 255–262 (2000).
Carta, L. et al. Engineering of macrophages to produce IFN-γ in response to hypoxia. J. Immunol. 166, 5374–5380 (2001).
Pastorino, S., Massazza, S., Cilli, M., Varesio, L. & Bosco, M.C. Generation of high-titer retroviral vector-producing macrophages as vehicles for in vivo gene transfer. Gene Ther. 8, 431–441 (2001).
Rosenberg, S.A. Adoptive immunotherapy of cancer: accomplishments and prospects. Cancer Treat. Rep. 68, 233–255 (1984).
Ioannides, C.G. & Whiteside, T.L. T cell recognition of human tumors: implications for molecular immunotherapy of cancer. Clin. Immunol. Immunopathol. 66, 91–106 (1993).
Melief, C.J. Tumor eradication by adoptive transfer of cytotoxic T lymphocytes. Adv. Cancer Res. 58, 143–175 (1992).
Whiteside, T.L. Monitoring of antigen-specific cytolytic T lymphocytes in cancer patients receiving immunotherapy. Clin. Diag. Lab. Immunol. 7, 327–332 (2000).
Yee, C. et al. Melanocyte destruction after antigen-specific immunotherapy of melanoma: direct evidence of T cell-mediated vitiligo. J. Exp. Med. 192, 1637–1644 (2000).
Yee, C., Riddell, S.R. & Greenberg, P.D. In vivo tracking of tumor-specific T cells. Curr. Opin. Immunol. 13, 141–146 (2001).
Di Carlo, E. et al. The intriguing role of polymorphonuclear neutrophils in antitumor reactions. Blood 97, 339–345 (2001).
Rosenberg, S.A. The immunotherapy and gene therapy of cancer. J. Clin. Onc. 10, 180–199 (1992).
Gomez-Navarro, J. et al. Genetically modified CD34+ cells as cellular vehicles for gene delivery into areas of angiogenesis in a rhesus model. Gene Ther. 7, 43–52 (2000).
Asahara, T. et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ. Res. 85, 221–228 (1999).
Boon, T. & van der Bruggen, P. Human tumor antigens recognized by T lymphocytes. J. Exp. Med. 183, 725–729 (1996).
Altenschmidt, U., Klundt, E. & Groner, B. Adoptive transfer of in vitro-targeted, activated T lymphocytes results in total tumor regression. J. Immunol. 159, 5509–5515 (1997).
Bolhuis, R.L. & Gratama, J.W. Genetic re-targeting of T lymphocyte specificity. Gene Ther. 5, 1153–1155 (1998).
Brocker, T., Riedinger, M. & Karjalainen, K. Redirecting the complete T cell receptor/CD3 signaling machinery towards native antigen via modified T cell receptor. Eur. J. Immunol. 26, 1770–1774 (1996).
Eshhar, Z., Waks, T., Gross, G. & Schindler, D.G. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the γ or ζ subunits of the immunoglobulin and T-cell receptors. Proc. Natl. Acad. Sci. USA 90, 720–724 (1993).
Hombach, A. et al. A chimeric receptor that selectively targets membrane-bound carcinoembryonic antigen (mCEA) in the presence of soluble CEA. Gene Ther. 6, 300–304 (1999).
Weijtens, M.E., Willemsen, R.A., Valerio, D., Stam, K. & Bolhuis, R.L. Single chain Ig/γ gene-redirected human T lymphocytes produce cytokines, specifically lyse tumor cells, and recycle lytic capacity. J. Immunol. 157, 836–843 (1996).
Alvarez-Vallina, L., Agha-Mohammadi, S., Hawkins, R.E. & Russell, S.J. Pharmacological control of antigen responsiveness in genetically modified T lymphocytes. J. Immunol. 159, 5889–5895 (1997).
Alvarez-Vallina, L., Yanez, R., Blanco, B., Gil, M. & Russell, S.J. Pharmacologic suppression of target cell recognition by engineered T cells expressing chimeric T-cell receptors. Cancer Gene Ther. 7, 526–529 (2000).
DiDonato, J. et al. Mapping of the inducible IκB phosphorylation sites that signal its ubiquitination and degradation. Mol. Cell Biol. 16, 1295–1304 (1996).
Sen, J. et al. Expression and induction of nuclear factor-κB-related proteins in thymocytes. J. Immunol. 154, 3213–3221 (1995).
Trushin, S.A., Pennington, K.N., Algeciras-Schimnich, A. & Paya, C.V. Protein kinase C and calcineurin synergize to activate IκB kinase and NF-κB in T lymphocytes. J. Biol. Chem. 274, 22923–22931 (1999).
Hollander, G.A. On the stochastic regulation of interleukin-2 transcription. Semin. Immunol. 11, 357–367 (1999).
Jain, J., Loh, C. & Rao, A. Transcriptional regulation of the IL-2 gene. Curr. Opin. Immunol. 7, 333–342 (1995).
Cantrell, D. T cell antigen receptor signal transduction pathways. Annu. Rev. Immunol. 14, 259–274 (1996).
Beg, A.A. et al. IκB interacts with the nuclear localization sequences of the subunits of NF-κB: a mechanism for cytoplasmic retention. Genes Dev. 6, 1899–1913 (1992).
Finzi, D. et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 278, 1295–1300 (1997).
McElhinny, J.A. et al. Regulation of IκB α and p105 in monocytes and macrophages persistently infected with human immunodeficiency virus. J. Virol. 69, 1500–1509 (1995).
Duisit, G., Salvetti, A., Moullier, P. & Cosset, F.L. Functional characterization of adenoviral/retroviral chimeric vectors and their use for efficient screening of retroviral producer cell lines. Hum. Gene Ther. 10, 189–200 (1999).
Morgenstern, J.P. & Land, H. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 18, 3587–3596 (1990).
Diaz, R.M., Eisen, T., Hart, I.R. & Vile, R.G. Exchange of viral promoter/enhancer elements with heterologous regulatory sequences generates targeted hybrid long terminal repeat vectors for gene therapy of melanoma. J. Virol. 72, 789–795 (1998).
Emiliusen, L. et al. A transcriptional feedback loop for tissue-specific expression of highly cytotoxic genes which incorporates an immunostimulatory component. Gene Ther. 8, 987–998 (2001).
Richards, C.A., Austin, E.A. & Huber, B.E. Transcriptional regulatory sequences of carcinoembryonic antigen: identification and use with cytosine deaminase for tumor-specific gene therapy. Hum. Gene Ther. 6, 881–893 (1995).
Wagner, M.J., Sharp, J.A. & Summers, W.C. Nucleotide sequence of the thymidine kinase gene of herpes simplex virus type 1. Proc. Natl. Acad. Sci. USA 78, 1441–1445 (1981).
Vile, R.G., Nelson, J.A., Castleden, S., Chong, H. & Hart, I.R. Systemic gene therapy of murine melanoma using tissue-specific expression of the HSVtk gene involves an immune component. Cancer Res. 54, 6228–6234 (1994).
Freeman, S.M. et al. The “bystander effect”: tumor regression when a fraction of the tumor mass is genetically modified. Cancer Res. 53, 5274–5283 (1993).
Alvarez-Vallina, L. & Russell, S. Efficient discrimination between different densities of target antigen by tetracycline-regulatable T bodies. Hum. Gene Ther. 10, 559–563 (1999).
Patel, S.D. et al. Impact of chimeric immune receptor extracellular protein domains on T cell function. Gene Ther. 6, 412–419 (1999).
Miller, D.G., Adam, M.A. & Miller, A.D. Gene transfer by retrovirus vectors occurs only in cells that are actively replicating at the time of infection. Mol. Cell. Biol. 10, 4239–4242 (1990).
Culver, K.W. et al. In vivo gene transfer with retroviral vector-producer cells for treatment of experimental brain tumors. Science 256, 1550–1552 (1992).
Naldini, L. et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 272, 263–266 (1996).
Zufferey, R., Nagy, D., Mandel, R.J., Naldini, L. & Trono, D. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat. Biotechnol. 15, 871–875 (1997).
Acknowledgements
The authors thank Toni L. Higgins for expert secretarial assistance. This work was supported by the Mayo Foundation and by the Association of Cancer Physicians of the United Kingdom (J.C). We are grateful to Dr. Carlos Paya of the Mayo Clinic for supplying the (NF-κB)3 promoter fragment.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Chester, J., Ruchatz, A., Gough, M. et al. Tumor antigen–specific induction of transcriptionally targeted retroviral vectors from chimeric immune receptor–modified T cells. Nat Biotechnol 20, 256–263 (2002). https://doi.org/10.1038/nbt0302-256
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nbt0302-256
This article is cited by
-
Antitumor efficacy of oncolytic herpes simplex virus adsorbed onto antigen-specific lymphocytes
Cancer Gene Therapy (2012)
-
Cell Carriers for Oncolytic Viruses: Fed Ex for Cancer Therapy
Molecular Therapy (2009)
-
Cell vehicle targeting strategies
Gene Therapy (2008)
-
Purging metastases in lymphoid organs using a combination of antigen-nonspecific adoptive T cell therapy, oncolytic virotherapy and immunotherapy
Nature Medicine (2008)
-
Loading of oncolytic vesicular stomatitis virus onto antigen-specific T cells enhances the efficacy of adoptive T-cell therapy of tumors
Gene Therapy (2008)