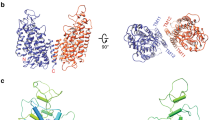
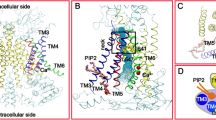
Abstract
Calcium-activated chloride channels (CaCCs) encoded by TMEM16A1,2,3 control neuronal signalling, smooth muscle contraction, airway and exocrine gland secretion, and rhythmic movements of the gastrointestinal system4,5,6,7. To understand how CaCCs mediate and control anion permeation to fulfil these physiological functions, knowledge of the mammalian TMEM16A structure and identification of its pore-lining residues are essential. TMEM16A forms a dimer with two pores8,9. Previous CaCC structural analyses have relied on homology modelling of a homologue (nhTMEM16) from the fungus Nectria haematococca that functions primarily as a lipid scramblase10,11,12, as well as subnanometre-resolution electron cryo-microscopy12. Here we present de novo atomic structures of the transmembrane domains of mouse TMEM16A in nanodiscs and in lauryl maltose neopentyl glycol as determined by single-particle electron cryo-microscopy. These structures reveal the ion permeation pore and represent different functional states. The structure in lauryl maltose neopentyl glycol has one Ca2+ ion resolved within each monomer with a constricted pore; this is likely to correspond to a closed state, because a CaCC with a single Ca2+ occupancy requires membrane depolarization in order to open (C.J.P. et al., manuscript submitted). The structure in nanodiscs has two Ca2+ ions per monomer and its pore is in a closed conformation; this probably reflects channel rundown, which is the gradual loss of channel activity that follows prolonged CaCC activation in 1 mM Ca2+. Our mutagenesis and electrophysiological studies, prompted by analyses of the structures, identified ten residues distributed along the pore that interact with permeant anions and affect anion selectivity, as well as seven pore-lining residues that cluster near pore constrictions and regulate channel gating. Together, these results clarify the basis of CaCC anion conduction.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Caputo, A. et al. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science 322, 590–594 (2008)
Schroeder, B. C., Cheng, T., Jan, Y. N. & Jan, L. Y. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell 134, 1019–1029 (2008)
Yang, Y. D. et al. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature 455, 1210–1215 (2008)
Hartzell, C., Putzier, I. & Arreola, J. Calcium-activated chloride channels. Annu. Rev. Physiol. 67, 719–758 (2005)
Huang, F., Wong, X. & Jan, L. Y. International Union of Basic and Clinical Pharmacology. LXXXV: calcium-activated chloride channels. Pharmacol. Rev. 64, 1–15 (2012)
Pedemonte, N. & Galietta, L. J. Structure and function of TMEM16 proteins (anoctamins). Physiol. Rev. 94, 419–459 (2014)
Oh, U. & Jung, J. Cellular functions of TMEM16/anoctamin. Pflügers Arch. 468, 443–453 (2016)
Lim, N. K., Lam, A. K. & Dutzler, R. Independent activation of ion conduction pores in the double-barreled calcium-activated chloride channel TMEM16A. J. Gen. Physiol. 148, 375–392 (2016)
Jeng, G., Aggarwal, M., Yu, W. P. & Chen, T. Y. Independent activation of distinct pores in dimeric TMEM16A channels. J. Gen. Physiol. 148, 393–404 (2016)
Brunner, J. D., Lim, N. K., Schenck, S., Duerst, A. & Dutzler, R. X-ray structure of a calcium-activated TMEM16 lipid scramblase. Nature 516, 207–212 (2014)
Brunner, J. D., Schenck, S. & Dutzler, R. Structural basis for phospholipid scrambling in the TMEM16 family. Curr. Opin. Struct. Biol. 39, 61–70 (2016)
Paulino, C. et al. Structural basis for anion conduction in the calcium-activated chloride channel TMEM16A. eLife 6, e26232 (2017)
Scudieri, P., Sondo, E., Caci, E., Ravazzolo, R. & Galietta, L. J. TMEM16A–TMEM16B chimaeras to investigate the structure–function relationship of calcium-activated chloride channels. Biochem. J. 452, 443–455 (2013)
Scudieri, P., Musante, I., Gianotti, A., Moran, O. & Galietta, L. J. Intermolecular interactions in the TMEM16A dimer controlling channel activity. Sci. Rep. 6, 38788 (2016)
Alexandrov, A. I., Mileni, M., Chien, E. Y., Hanson, M. A. & Stevens, R. C. Microscale fluorescent thermal stability assay for membrane proteins. Structure 16, 351–359 (2008)
Ashok, Y. & Jaakola, V. P. Nanodisc-Tm: rapid functional assessment of nanodisc reconstituted membrane proteins by CPM assay. MethodsX 3, 212–218 (2016)
Duriseti, S. et al. Antagonistic anti-urokinase plasminogen activator receptor (uPAR) antibodies significantly inhibit uPAR-mediated cellular signaling and migration. J. Biol. Chem. 285, 26878–26888 (2010)
Wu, S. et al. Fabs enable single particle cryoEM studies of small proteins. Structure 20, 582–592 (2012)
Smart, O. S., Neduvelil, J. G., Wang, X., Wallace, B. A. & Sansom, M. S. HOLE: a program for the analysis of the pore dimensions of ion channel structural models. J. Mol. Graph. 14, 354–360 (1996)
Whitlock, J. M. & Hartzell, H. C. A pore idea: the ion conduction pathway of TMEM16/ANO proteins is composed partly of lipid. Pflügers Arch. 468, 455–473 (2016)
Whitlock, J. M. & Hartzell, H. C. Anoctamins/TMEM16 proteins: chloride channels flirting with lipids and extracellular vesicles. Annu. Rev. Physiol. 79, 119–143 (2017)
Yu, K. et al. Identification of a lipid scrambling domain in ANO6/TMEM16F. eLife 4, e06901 (2015)
Jiang, T., Yu, K., Hartzell, H. C. & Tajkhorshid, E. Lipids and ions traverse the membrane by the same physical pathway in the nhTMEM16 scramblase. eLife 6, e28671 (2017)
Peters, C. J. et al. Four basic residues critical for the ion selectivity and pore blocker sensitivity of TMEM16A calcium-activated chloride channels. Proc. Natl Acad. Sci. USA 112, 3547–3552 (2015)
Tien, J. et al. A comprehensive search for calcium binding sites critical for TMEM16A calcium-activated chloride channel activity. eLife 3, e02772 (2014)
Yu, K., Duran, C., Qu, Z., Cui, Y. Y. & Hartzell, H. C. Explaining calcium-dependent gating of anoctamin-1 chloride channels requires a revised topology. Circ. Res. 110, 990–999 (2012)
Yang, H. et al. TMEM16F forms a Ca2+-activated cation channel required for lipid scrambling in platelets during blood coagulation. Cell 151, 111–122 (2012)
Goehring, A. et al. Screening and large-scale expression of membrane proteins in mammalian cells for structural studies. Nat. Protoc. 9, 2574–2585 (2014)
Whicher, J. R. & MacKinnon, R. Structure of the voltage-gated K+ channel Eag1 reveals an alternative voltage sensing mechanism. Science 353, 664–669 (2016)
Gao, Y., Cao, E., Julius, D. & Cheng, Y. TRPV1 structures in nanodiscs reveal mechanisms of ligand and lipid action. Nature 534, 347–351 (2016)
Mastronarde, D. N. Automated electron microscope tomography using robust prediction of specimen movements. J. Struct. Biol. 152, 36–51 (2005)
Liao, M., Cao, E., Julius, D. & Cheng, Y. Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature 504, 107–112 (2013)
Frank, J. et al. SPIDER and WEB: processing and visualization of images in 3D electron microscopy and related fields. J. Struct. Biol. 116, 190–199 (1996)
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017)
Rohou, A. & Grigorieff, N. CTFFIND4: Fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221 (2015)
Zhang, K. Gctf: Real-time CTF determination and correction. J. Struct. Biol. 193, 1–12 (2016)
Scheres, S. H. RELION: implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol. 180, 519–530 (2012)
Tang, G. et al. EMAN2: an extensible image processing suite for electron microscopy. J. Struct. Biol. 157, 38–46 (2007)
Scheres, S. H. & Chen, S. Prevention of overfitting in cryo-EM structure determination. Nat. Methods 9, 853–854 (2012)
Kucukelbir, A., Sigworth, F. J. & Tagare, H. D. Quantifying the local resolution of cryo-EM density maps. Nat. Methods 11, 63–65 (2014)
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D 66, 486–501 (2010)
Trabuco, L. G., Villa, E., Mitra, K., Frank, J. & Schulten, K. Flexible fitting of atomic structures into electron microscopy maps using molecular dynamics. Structure 16, 673–683 (2008)
Adams, P. D . et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D 66, 213–221 (2010)
Brown, A. et al. Tools for macromolecular model building and refinement into electron cryo-microscopy reconstructions. Acta Crystallogr. D 71, 136–153 (2015)
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D 66, 12–21 (2010)
Barad, B. A. et al. EMRinger: side chain-directed model and map validation for 3D cryo-electron microscopy. Nat. Methods 12, 943–946 (2015)
DeLano, W. L. The PyMOL molecular graphics system on World Wide Web. http://www.pymol.org (2002)
Saxton, W. O. & Baumeister, W. The correlation averaging of a regularly arranged bacterial cell envelope protein. J. Microsc. 127, 127–138 (1982)
van Heel, M. & Stöffler-Meilicke, M. Characteristic views of E. coli and B. stearothermophilus 30S ribosomal subunits in the electron microscope. EMBO J. 4, 2389–2395 (1985)
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004)
Rosenthal, P. B. & Henderson, R. Optimal determination of particle orientation, absolute hand, and contrast loss in single-particle electron cryomicroscopy. J. Mol. Biol. 333, 721–745 (2003)
Wright, S. P. Adjusted P-values for simultaneous inference. Biometrics 48, 1005–1013 (1992)
Acknowledgements
We thank R. MacKinnon, T. Xiao and W. Wang for advice on protein purification; B. Kobilka and A. Koehl for help with the CPM assay; Z. Yu and his colleagues at the HHMI Janelia Cryo-EM Facility for help with data acquisition; J. Wang for discussions on model building; P. Jin for help with data presentation; and colleagues in our laboratories for discussion. This work was supported by grants from the NIH (R01GM098672 and S100D0020054 to Y.C., R01NS069229 to L.Y.J., R35NS097227 to Y.N.J., R01HL080050 and R01DC007664 to D.L.M., P41CA196276 and P50GM111126 to C.S.C., K99DA041500 to C.J.P.) and from the American Heart Association (M.L.), and by UCSF Breakthrough Biomedical Research (Y.C.). S.D. is supported by a Human Frontier Science Program (HFSP) Postdoctoral Fellowship. J.Z. is supported by a Banting Postdoctoral Fellowship from the Canadian Institute of Health Research. L.Y.J., Y.N.J. and Y.C. are Investigators with the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
S.D., S.F. and J.T. designed and performed most of the biochemical and cryo-EM experiments. C.J.P., J.T., W.Y., L.Q. and T.C. performed mutagenesis and electrophysiology experiments. K.Z. and J.T. isolated the Fab that binds TMEM16A. M.L. and J.T. performed systematic screens of deletion constructs and purification schemes. S.D., D.B., M.L. and S.F. built de novo models. J.Z. developed methods to calculate directional resolution. L.Y.J., Y.N.J., D.L.M., C.S.C. and Y.C. supervised experiments and data analysis. All authors contributed to manuscript preparation.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reviewer Information Nature thanks H. C. Hartzell, S. Newstead and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Figure 1 TMEM16A protein purification and negative staining.
a, Western blot (bottom) of nine TMEM16A constructs with different N-terminal and/or C-terminal truncations (diagrammed, top). Blue, wild-type (WT) TMEM16A and constructs with N-terminal truncations. Orange, TMEM16A constructs with additional C-terminal truncation. Construct 5, corresponding to mouse TMEM16A residues 1–903, was selected for this study because of its high expression and the absence of the smaller fragment of ~30 kD on the western blot. b, Top, representative trace of inside-out patch from HEK293 cells transiently transfected with wild-type TMEM16A or construct 5. The membrane potential was held at 60 mV, and patches were exposed to intracellular solutions that contained 140 mM NaCl and 150 nM, 300 nM, 400 nM, 600 nM, 1.8 μM or 1 mM free Ca2+. The experiment was repeated independently four times with similar results. Bottom, normalized chloride currents were fit to the Hill equation. EC50 for Ca2+ sensitivity is 178 ± 14 nM for construct 5 (four independent experiments) and 796 ± 66 nM for wild type (ten independent experiments; P < 0.0001, see Extended Data Fig. 9c). c, Top, poly-l-lysine (PLL, 30 μg ml−1) treatment for 30 s, to reduce PIP2 and other lipids with negatively charged head groups, caused desensitization of TMEM16A with C-terminal truncation (amino acids 1–903) in an excised inside-out patch exposed to 150 mM NaCl on both sides of the membrane, as evident from the reduction of Ca2+ sensitivity. The experiment was repeated independently six times with similar results. Bottom, after PLL treatment, current amplitudes were reduced at 30 nM Ca2+ and 100 nM Ca2+. ‘Inst’, ‘instantaneous’ current amplitude at the start of depolarization from a holding potential of 0 mV–100 mV (P = 0.02 from two-way ANOVA between ‘pre’ and ‘post’ PLL); ‘overall’, current amplitude at the end of depolarization (P = 0.004 from two-way ANOVA between ‘pre’ and ‘post’; 6 independent experiments) but not at 1 μM Ca2+ (Sidak’s multiple comparisons, P > 0.99 and P = 0.73 for ‘inst’ and ‘overall’, respectively). Mean ± s.e.m. are shown in b and c. d, Size-exclusion chromatography of TMEM16A reconstituted in lipid nanodiscs with MSP2N2. The peak fractions corresponding to TMEM16A reconstituted in nanodiscs (16A) and free MSP2N2 are indicated. The 16A peak fraction was examined by SDS–polyacrylamide gel electrophoresis (SDS–PAGE). The TMEM16A and MSP2N2 (MSP) monomers are approximately 105 kDa and 46 kDa, respectively. The faint band at 210 kDa may correspond to incompletely disassociated TMEM16A dimers. e, CPM analysis15,16 of TMEM16A reconstituted in nanodiscs, in 0, 71 nM, 293 nM, 782 nM, 4,120 nM or 1 mM Ca2+. f, Raw micrographs of TMEM16A reconstituted in nanodiscs, examined by negative-stain electron microscopy. g, 2D-class averages of particles from negative-stain electron microscopy of TMEM16A reconstituted in nanodiscs. h, Size-exclusion chromatography of TMEM16A solubilized in LMNG. The peak fraction was examined by SDS–PAGE. i, CPM analysis of TMEM16A solubilized in LMNG, in 0, 83 nM, 333 nM, 1,122 nM, 5,290 nM or 1 mM Ca2+. j, Raw micrographs of TMEM16A solubilized in LMNG, examined by negative-stain electron microscopy. Both micrographs (f, j) show mono-dispersed and homogeneous particles. k, 2D-class averages of particles from negative-stain electron microscopy of TMEM16A solubilized in LMNG.
Extended Data Figure 2 Cryo-EM analysis of TMEM16A reconstituted in nanodiscs.
a, A representative cryo-EM micrograph of TMEM16A reconstituted in nanodiscs. Green circles indicate individual particles. b, Representative 2D-class averages from boxed particles with 256-pixel box size (261.12 Å). c, Euler angle distribution of all particles included in the final 3D reconstruction. The size of the spheres is proportional to the number of particles seen from that specific orientation. d, FSC curves of two independently refined maps before (blue) and after (red) post-processing in RELION. Curves with resolution corresponding to FSC = 0.143 are shown. e, Planar slices through the unsharpened electron microscopy density map at different levels along the channel symmetry axis. f, Local resolution of TMEM16A as estimated by RELION, shown with pseudo-colour representation of resolution. g, Cross-validation using FSC curves of the density map calculated from the refined model versus half-map 1 (work), half-map 2 (free) and the summed map. h, dFSC from different Fourier cones. Each curve indicates a different direction. i, Calculated resolution from different views. The directions are indicated as x, y, and z in the 3D resolution map. The highest and lowest resolutions are labelled with red and blue circles, respectively. The green circle shows global average resolution.
Extended Data Figure 3 Cryo-EM analysis of TMEM16A solubilized in LMNG.
a, A representative cryo-EM micrograph of TMEM16A solubilized in LMNG. Green circles indicate individual particles. b, Representative 2D-class averages from boxed particles with a 256-pixel box size (261.12 Å) and TMEM16A in complex with Fabs (bottom row; the two right panels show particles after subtraction of densities for Fabs). c, Euler angle distribution of all particles included in the final 3D reconstruction. The size of the spheres is proportional to the number of particles visualized from that specific orientation. d, FSC curves of two independently refined maps before (blue) and after (red) post-processing in RELION. Curves with resolution corresponding to FSC = 0.143 are shown. e, Planar slices through the unsharpened electron microscopy density map at different levels along the channel symmetry axis. f, Local resolution of TMEM16A as estimated by RELION, shown with pseudo-colour representation of resolution. g, Cross-validation using FSC curves of the density map calculated from the refined model versus half-map 1 (work), half-map 2 (free) and the summed map. h, dFSC from different Fourier cones. Each curve indicates a different direction. dFSC for TMEM16A alone in LMNG in grey (average in yellow); dFSC for a combination of TMEM16A alone and Fabs-bound TMEM16A, in LMNG, shown in purple (average in red). i, Calculated resolution from different views (grey for combination of TMEM16A alone and Fabs-bound TMEM16A, yellow for TMEM16A alone). The directions are indicated as x, y, and z in the 3D resolution map. The highest and lowest resolutions are labelled with red and blue circles, respectively. The green circle shows global average resolution.
Extended Data Figure 4 Cryo-EM densities of the ten transmembrane helices of TMEM16A, summary of cryo-EM data collection, and processing and summaries of sidechain assignments.
a, Representative cryo-EM densities of the ten transmembrane helices (TM1–TM10) of TMEM16A reconstituted in nanodiscs (right) or TMEM16A solubilized in LMNG (left) are superimposed on the corresponding atomic model. The electron microscopy densities are shown in blue meshes for TMEM16A reconstituted in nanodiscs, or green meshes for TMEM16A solubilized in LMNG. The model is shown as sticks coloured according to atom type: C, light grey; N, blue; O, red; and S, yellow. b, Summary of cryo-EM data collection and model refinement. c, Summary of sidechain assignment of TMEM16A in nanodiscs. d, Summary of sidechain assignment of TMEM16A in LMNG.
Extended Data Figure 5 Atomic models of TMEM16A in two conformations.
a–c, Ribbon diagrams of TMEM16A reconstituted in nanodiscs (in green and yellow) with lipids (in red), overlaid on the electron density map (sharpened, in light grey). Two Ca2+ ions (orange spheres) are present in each monomer. d–f, Ribbon diagrams of TMEM16A solubilized in LMNG (in blue) with lipids (in red), overlaid on the electron density map (sharpened, in light grey). One Ca2+ ion (orange sphere) is present in each monomer. g–i, Electron densities of TMEM16A reconstituted in nanodiscs (unsharpened, in green and yellow) overlaid on TMEM16A solubilized in digitonin12 (in grey).
Extended Data Figure 6 Anion selectivity depends on residues lining the pore surrounded by TM3–8 but not TM10 residues at the dimer interface.
a, Bi-ionic conditions for assessing the effect of the V595L mutation on permeability ratios. b, Effects of different substitutions of V595 on the permeability ratio  (2.70 ± 0.09, n = 28 for WT; 4.00 ± 0.16, n = 9 for V595A; 3.49 ± 0.21, n = 6 for V595K; 3.81 ± 0.07, n = 7 for V595L; 3.48 ± 0.14, n = 7 for V595R). c, Effects of different substitutions of V595 on the permeability ratio
(2.70 ± 0.09, n = 28 for WT; 4.00 ± 0.16, n = 9 for V595A; 3.49 ± 0.21, n = 6 for V595K; 3.81 ± 0.07, n = 7 for V595L; 3.48 ± 0.14, n = 7 for V595R). c, Effects of different substitutions of V595 on the permeability ratio  (5.47 ± 0.21, n = 28 for WT; 9.96 ± 0.30, n = 9 for V595A; 6.64 ± 0.58, n = 6 for V595K; 7.86 ± 0.37, n = 7 for V595L; 6.30 ± 0.37, n = 7 for V595R). d, Permeability ratios determined in bi-ionic conditions for TMEM16A mutants. The exact n values (independent experimental samples from individually recorded HEK293 cells) are given for every experiment. The P values were generated with a Dunnett’s post hoc test after a one-way ANOVA. For these multiplicity adjusted P values, values smaller than 0.0001 cannot be estimated precisely; Prism’s documentation suggests this approach is the most rigorous and conservative way to generate a P value from a multiple comparison test52.
(5.47 ± 0.21, n = 28 for WT; 9.96 ± 0.30, n = 9 for V595A; 6.64 ± 0.58, n = 6 for V595K; 7.86 ± 0.37, n = 7 for V595L; 6.30 ± 0.37, n = 7 for V595R). d, Permeability ratios determined in bi-ionic conditions for TMEM16A mutants. The exact n values (independent experimental samples from individually recorded HEK293 cells) are given for every experiment. The P values were generated with a Dunnett’s post hoc test after a one-way ANOVA. For these multiplicity adjusted P values, values smaller than 0.0001 cannot be estimated precisely; Prism’s documentation suggests this approach is the most rigorous and conservative way to generate a P value from a multiple comparison test52.
Extended Data Figure 7 Comparisons of extracellular loops and lipids in TMEM16a reconstituted in nanodiscs and TMEM16A solubilized in LMNG.
a–c, Lipids (in red) in TMEM16A reconstituted in nanodiscs (in green and yellow, overlaid on the electron density map in light grey) (b, c), with helical distortions of TM6 near G640 (a). d–f, Lipids (in red) in TMEM16A solubilized in LMNG (in blue, overlaid on the electron density map in light grey) (e, f); the lower half of TM6 beyond G640 is disordered and is therefore absent from the reconstruction (d). g–i, Extracellular domains of TMEM16A reconstituted in nanodiscs (unsharpened, in green and yellow) overlaid on those of TMEM16A solubilized in LMNG (unsharpened, in blue). j–l, Extracellular TM5–TM6 and TM9–TM10 loops in ribbon diagrams for TMEM16A reconstituted in nanodiscs (in green and yellow) overlaid on those of TMEM16A solubilized in LMNG (in blue).
Extended Data Figure 8 Data processing of TMEM16A in nanodiscs or TMEM16A in LMNG.
a, Data processing of TMEM16A reconstituted in nanodiscs. Particle picking was performed with Gautomatch with templates from 2D classes from the LMNG dataset, which generated 927,414 particles in total. All particles were extracted and binned in groups of four (pixel size of 4.08 Å) and then 2D classified. For 3D refinement, 341,875 particles from good 2D classes were used, with an initial model from the LMNG structure low-pass filtered to 60-Å resolution; this produced a 5.5-Å resolution map. The 5.5-Å resolution map was then low-pass filtered to 10 Å, as the initial model for 3D classification without applied symmetry for all particles with a 1.02-Å pixel size. Of the seven classes, two classes (15.92% and 11.15% of the 927,414 particles) gave maps with an improved resolution (4.7 Å and 5.1 Å, respectively) after 3D auto-refinement with C2 symmetry. These two classes were combined together, for a total of 251,851 particles, and we ran another 3D auto-refinement to generate the unmasked map at a resolution of 4.6 Å. The map was then masked to get the final map at a resolution of 3.8 Å. b, Data processing of TMEM16A solubilized in LMNG. Approximately 4,000 particles were manually picked and classified by 2D classification in SAMUEL to generate the templates for automatic particle picking with samautopick.py. After manual inspection, 533,545 particles were identified. The crystal structure of nhTMEM16A10 (PDB: 4WIS) was converted to .mrc format with e2pdb2mrc.py and low-pass filtered to a resolution of 60 Å to produce the initial model. Selected 2D classes (44 out of 200) were used for 3D auto-refinement with C2 symmetry. Because 3D classification failed to produce further separation, particles from all five classes were used to generate the final map with a resolution of 3.8 Å. To reduce the anisotropy that was the result of the underrepresentation of side views, this dataset was merged with another dataset, which was derived from Fabs bound to TMEM16A in LMNG. Starting with 338,705 particles from automatic particle picking, 4 of 40 2D classes (132,444 particles) were used for 3D auto-refinement. The Fab density for each particle was then subtracted. The 132,444 subtracted particles without Fab density were combined with the 342,875 particles from all 5 classes of the TMEM16A in LMNG dataset with a resolution of 3.8 Å, and processed with 3D auto-refinement to generate the unmasked map with a resolution of 3.9 Å. This map was then masked to get the final map at resolution of 3.4 Å. Pixel sizes are shown in parentheses for each class.
Extended Data Figure 9 Multiple open and closed states of the TMEM16A CaCC and the involvement of pore-lining residues in channel gating.
a, A decrease from 150 mM NaCl to 15 mM NaCl in the intracellular solution, which contained 1 μM or 1 mM Ca2+, caused an identical shift in the reversal potential of wild-type TMEM16A (WT), but not K584Q mutant, channels in an excised inside-out patch held at 80 mV and subjected to a ramp to −80 mV. Experiment was repeated independently 8 times for wild type with similar results, and 5 times for K584Q with similar results. b, The K584Q mutation altered the permeability ratio  at 1 μM but not at 1 mM Ca2+. n = 8 for wild type, n = 5 for K584Q. c, Calcium sensitivity of channel activation of wild-type and mutant TMEM16A channels (number of independent experiments and P values are given in the table). Permeability ratios for mutants were compared to those of wild type using one-way ANOVA followed by the Bonferroni post hoc test for significance; ****P < 0.0001, ***P < 0.001, **P < 0.005, data are mean ± s.e.m.
at 1 μM but not at 1 mM Ca2+. n = 8 for wild type, n = 5 for K584Q. c, Calcium sensitivity of channel activation of wild-type and mutant TMEM16A channels (number of independent experiments and P values are given in the table). Permeability ratios for mutants were compared to those of wild type using one-way ANOVA followed by the Bonferroni post hoc test for significance; ****P < 0.0001, ***P < 0.001, **P < 0.005, data are mean ± s.e.m.
Extended Data Figure 10 Sequence alignment of TMEM16A homologues.
a, Sequences of TMEM16A homologues were analysed by Clustal Omega. Conserved residues are highlighted. Transmembrane helices are indicated above the sequences. Residues shown to be crucial for selectivity in this (D550, N587, S635, Q705 and F712) and previous (R511, K584 and K599) studies are marked in orange and blue, respectively. Residues (I546, Y589, I592, L639 and F708) shown in this study to be critical for gating are marked in green. Residues (N542 and V595) that contributed to both selectivity and gating properties are marked in purple. The residues (E650, E698, E701, E730 and D734) that are important for Ca2+ binding are marked in red. b, Sequence alignment of mouse TMEM16A and nhTMEM16. Conserved residues are highlighted. Transmembrane helices of TMEM16A are indicated above the sequence.
Supplementary information
Rights and permissions
About this article
Cite this article
Dang, S., Feng, S., Tien, J. et al. Cryo-EM structures of the TMEM16A calcium-activated chloride channel. Nature 552, 426–429 (2017). https://doi.org/10.1038/nature25024
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature25024
This article is cited by
-
Transmembrane proteins with unknown function (TMEMs) as ion channels: electrophysiological properties, structure, and pathophysiological roles
Experimental & Molecular Medicine (2024)
-
Structural heterogeneity of the ion and lipid channel TMEM16F
Nature Communications (2024)
-
Cryo-EM structure of TMEM63C suggests it functions as a monomer
Nature Communications (2023)
-
A mechanical-coupling mechanism in OSCA/TMEM63 channel mechanosensitivity
Nature Communications (2023)
-
The ϕPA3 phage nucleus is enclosed by a self-assembling 2D crystalline lattice
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.