Abstract
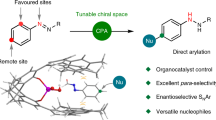
The directed activation of carbon–hydrogen bonds (C–H) is important in the development of synthetically useful reactions, owing to the proximity-induced reactivity and selectivity that is enabled by coordinating functional groups1,2,3,4,5,6. Palladium-catalysed non-directed C–H activation could potentially enable further useful reactions, because it can reach more distant sites and be applied to substrates that do not contain appropriate directing groups; however, its development has faced substantial challenges associated with the lack of sufficiently active palladium catalysts7,8. Currently used palladium catalysts are reactive only with electron-rich arenes, unless an excess of arene is used9,10,11,12,13,14,15,16,17,18, which limits synthetic applications. Here we report a 2-pyridone ligand that binds to palladium and accelerates non-directed C–H functionalization with arene as the limiting reagent. This protocol is compatible with a broad range of aromatic substrates and we demonstrate direct functionalization of advanced synthetic intermediates, drug molecules and natural products that cannot be used in excessive quantities. We also developed C–H olefination and carboxylation protocols, demonstrating the applicability of our methodology to other transformations. The site selectivity in these transformations is governed by a combination of steric and electronic effects, with the pyridone ligand enhancing the influence of sterics on the selectivity, thus providing complementary selectivity to directed C–H functionalization.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Whisler, M. C., MacNeil, S., Snieckus, V. & Beak, P. Beyond thermodynamic acidity: a perspective on the complex-induced proximity effect (CIPE) in deprotonation reactions. Angew. Chem. Int. Ed. 43, 2206–2225 (2004)
Kakiuchi, F. et al. Catalytic addition of aromatic carbon–hydrogen bonds to olefins with the aid of ruthenium complexes. Bull. Chem. Soc. Jpn 68, 62–83 (1995)
Daugulis, O., Do, H.-Q. & Shabashov, D. Palladium- and copper-catalyzed arylation of carbon–hydrogen bonds. Acc. Chem. Res. 42, 1074–1086 (2009)
Engle, K. M., Mei, T.-S., Wasa, M. & Yu, J.-Q. Weak coordination as powerful means for developing broadly useful C–H functionalization reactions. Acc. Chem. Res. 45, 788–802 (2012)
Lyons, T. W. & Sanford, M. S. Palladium-catalyzed ligand-directed C–H functionalization reactions. Chem. Rev. 110, 1147–1169 (2010)
Colby, D. A., Bergman, R. G. & Ellman, J. A. Rhodium-catalyzed C–C bond formation via heteroatom-directed C–H bond activation. Chem. Rev. 110, 624–655 (2010)
Kuhl, N., Hopkinson, M. N., Wencel-Delord, J. & Glorius, F. Beyond directing groups: transition-metal-catalyzed C–H activation of simple arenes. Angew. Chem. Int. Ed. 51, 10236–10254 (2012)
Hartwig, J. F. & Larsen, M. A. Undirected, homogeneous C–H bond functionalization: challenges and opportunities. ACS Cent. Sci. 2, 281–292 (2016)
Moritani, I. & Fujiwara, Y. Aromatic substitution of styrene-palladium chloride complex. Tetrahedr. Lett. 8, 1119–1122 (1967)
Dams, M., De Vos, D. E., Celen, S. & Jacobs, P. A. Toward waste-free production of Heck products with a catalytic palladium system under oxygen. Angew. Chem. Int. Ed. 42, 3512–3515 (2003)
Yokota, T., Tani, M., Sakaguchi, S. & Ishii, Y. Direct coupling of benzene with olefin catalyzed by Pd(OAc)2 combined with heteropolyoxometalate under dioxygen. J. Am. Chem. Soc. 125, 1476–1477 (2003)
Zhang, Y.-H., Shi, B.-F. & Yu, J.-Q. Pd(II)-catalyzed olefination of electron-deficient arenes using 2,6-dialkylpyridine ligands. J. Am. Chem. Soc. 131, 5072–5074 (2009)
Kubota, A., Emmert, M. H. & Sanford, M. S. Pyridine ligands as promoters in PdII/0-catalyzed C–H olefination reactions. Org. Lett. 14, 1760–1763 (2012)
Ying, C.-H., Yan, S.-B. & Duan, W.-L. 2-Hydroxy-1,10-phenanthroline vs 1,10-phenanthroline: significant ligand acceleration effects in the palladium-catalyzed oxidative Heck reaction of arenes. Org. Lett. 16, 500–503 (2014)
Li, R., Jiang, L. & Lu, W. Intermolecular cross-coupling of simple arenes via C–H activation by tuning concentrations of arenes and TFA. Organometallics 25, 5973–5975 (2006)
Shrestha, R., Mukherjee, P., Tan, Y., Litman, Z. C. & Hartwig, J. F. Sterically controlled, palladium-catalyzed intermolecular amination of arenes. J. Am. Chem. Soc. 135, 8480–8483 (2013)
Fujiwara, Y., Taniguchi, H. & Taniguchi, H. Palladium-promoted one-step carboxylation of aromatic compounds with carbon monoxide. J. Chem. Soc. Chem. Commun. 220–221 (1980)
Yoneyama, T. & Crabtree, R. H. Pd(II) catalyzed acetoxylation of arenes with iodosyl acetate. J. Mol. Catal. A 108, 35–40 (1996)
Huang, C., Chattopadhyay, B. & Gevorgyan, V. Silanol: a traceless directing group for Pd-catalyzed o-alkenylation of phenols. J. Am. Chem. Soc. 133, 12406–12409 (2011)
Bedford, R. B., Coles, S. J., Hursthouse, M. B. & Limmert, M. E. The catalytic intermolecular orthoarylation of phenols. Angew. Chem. Int. Ed. 42, 112–114 (2003)
Zhang, F.-L., Hong, K., Li, T.-J., Park, H. & Yu, J.-Q. Functionalization of C(sp3)–H bonds using a transient directing group. Science 351, 252–256 (2016)
Engle, K. M. & Yu, J.-Q. Developing ligands for palladium(II)-catalyzed C–H functionalization: intimate dialogue between ligand and substrate. J. Org. Chem. 78, 8927–8955 (2013)
Leow, D., Li, G., Mei, T.-S. & Yu, J.-Q. Activation of remote meta-C–H bond assisted by an end-on template. Nature 486, 518–522 (2012)
Chu, L. et al. Remote meta-C–H activation using a pyridine-based template: achieving site-selectivity via the recognition of distance and geometry. ACS Cent. Sci. 1, 394–399 (2015)
Mkhalid, I. A. I., Barnard, J. H., Marder, T. B., Murphy, J. M. & Hartwig, J. F. C–H activation for the construction of C–B bonds. Chem. Rev. 110, 890–931 (2010)
Cho, J.-Y., Tse, M. K., Holmes, D., Maleczka, R. E. Jr & Smith, M. R. III. Remarkably selective iridium catalysts for the elaboration of aromatic C–H bonds. Science 295, 305–308 (2002)
Grimster, N. P., Gauntlett, C., Godfrey, C. R. A. & Gaunt, M. J. Palladium-catalyzed intermolecular alkenylation of indoles by solvent-controlled regioselective C–H functionalization. Angew. Chem. Int. Ed. 44, 3125–3129 (2005)
Ueda, K., Yanagisawa, S., Yamaguchi, J. & Itami, K. A general catalyst for the β-selective C–H Bond arylation of thiophenes with iodoarenes. Angew. Chem. Int. Ed. 49, 8946–8949 (2010)
Bedford, R. B. et al. Facile hydrolysis and alcoholysis of palladium acetate. Angew. Chem. Int. Ed. 54, 6591–6594 (2015)
Wang, P. et al. Ligand-promoted meta-C–H arylation of anilines, phenols, and heterocycles. J. Am. Chem. Soc. 138, 9269–9276 (2016)
Acknowledgements
We acknowledge The Scripps Research Institute, the NIH (NIGMS, 2R01 GM102265), Bristol-Myers Squibb and Shanghai RAAS Blood Products Co., Ltd. for their financial support. We also thank Novartis for providing the drug molecules.
Author information
Authors and Affiliations
Contributions
P.W. developed the ligands and the reactions. P.V. performed the DFT calculations. G.X. performed the kinetic study. J.S., S.T. and P.T.W.C. separated the isomers using preparative HPLC. J.X.Q. and M.A.P. participated in the screening of acrylamide-derived coupling partners and investigation of the C–H olefination reaction for amino acid substrates. M.E.F. performed preliminary studies on 2-hydroxypyridine ligands. K.-S.Y. helped with the screening of sulphonamide-derived coupling partners. J.-Q.Y. conceived the concept and prepared the manuscript with feedback from P.W., P.V. and G.X.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reviewer Information Nature thanks J. de Vries and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
This file contains Supplementary Information schemes S1-S5. (PDF 76894 kb)
Supplementary Data
This zip file contains the cif files and CheckCIF documents for X-ray structures. (ZIP 2016 kb)
Rights and permissions
About this article
Cite this article
Wang, P., Verma, P., Xia, G. et al. Ligand-accelerated non-directed C–H functionalization of arenes. Nature 551, 489–493 (2017). https://doi.org/10.1038/nature24632
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature24632
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.