Abstract
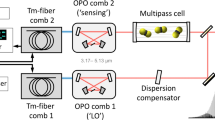
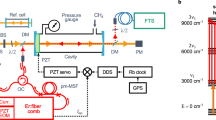
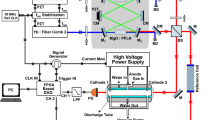
For more than half a century, high-resolution infrared spectroscopy has played a crucial role in probing molecular structure and dynamics. Such studies have so far been largely restricted to relatively small and simple systems, because at room temperature even molecules of modest size already occupy many millions of rotational/vibrational states, yielding highly congested spectra that are difficult to assign. Targeting more complex molecules requires methods that can record broadband infrared spectra (that is, spanning multiple vibrational bands) with both high resolution and high sensitivity. However, infrared spectroscopic techniques have hitherto been limited either by narrow bandwidth and long acquisition time1, or by low sensitivity and resolution2. Cavity-enhanced direct frequency comb spectroscopy (CE-DFCS) combines the inherent broad bandwidth and high resolution of an optical frequency comb with the high detection sensitivity provided by a high-finesse enhancement cavity3,4, but it still suffers from spectral congestion5. Here we show that this problem can be overcome by using buffer gas cooling6 to produce continuous, cold samples of molecules that are then subjected to CE-DFCS. This integration allows us to acquire a rotationally resolved direct absorption spectrum in the C–H stretching region of nitromethane, a model system that challenges our understanding of large-amplitude vibrational motion7,8,9. We have also used this technique on several large organic molecules that are of fundamental spectroscopic and astrochemical relevance, including naphthalene10, adamantane11 and hexamethylenetetramine12. These findings establish the value of our approach for studying much larger and more complex molecules than have been probed so far, enabling complex molecules and their kinetics to be studied with orders-of-magnitude improvements in efficiency, spectral resolution and specificity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Gagliardi, G. & Loock, H.-P. (eds) Cavity-Enhanced Spectroscopy and Sensing Chs 4–7 (Springer, 2014)
Griffith, P. R. & Haseth, J. A. Fourier Transform Infrared Spectrometry (Wiley, 2007)
Thorpe, M. J. et al. Broadband cavity ringdown spectroscopy for sensitive and rapid molecular detection. Science 311, 1595–1599 (2006)
Foltynowicz, A. et al. Cavity-enhanced optical frequency comb spectroscopy in the mid-infrared application to trace detection of hydrogen peroxide. Appl. Phys. B 110, 163–175 (2013)
Adler, F. et al. Mid-infrared Fourier transform spectroscopy with a broadband frequency comb. Opt. Express 18, 21861–21872 (2010)
Patterson, D., Tsikata, E. & Doyle, J. M. Cooling and collisions of large gas phase molecules. Phys. Chem. Chem. Phys. 12, 9736–9741 (2010)
Tannenbaum, E., Myers, R. J. & Gwinn, W. D. Microwave spectra, dipole moment, and barrier to internal rotation of CH3NO2 and CD3NO2 . J. Chem. Phys. 25, 42–47 (1956)
Sørensen, G. O. & Pedersen, T. Symmetry and microwave spectrum of nitromethane. Stud. Phys. Theor. Chem. 23, 219–236 (1983)
Dawadi, M. B. et al. High-resolution Fourier transform infrared synchrotron spectroscopy of the NO2 in-plane rock band of nitromethane. J. Mol. Spectrosc. 315, 10–15 (2015)
Albert, S. et al. Synchrotron-based highest resolution Fourier transform infrared spectroscopy of naphthalene (C10H8) and indole (C8H7N) and its application to astrophysical problems. Faraday Discuss. 150, 71–99 (2011)
Pirali, O. et al. Rotationally resolved infrared spectroscopy of adamantane. J. Chem. Phys. 136, 024310 (2012)
Pirali, O. & Boudon, V. Synchrotron-based Fourier transform spectra of the v23 and v24 IR bands of hexamethylenetetramine C6N4H12 . J. Mol. Spectrosc. 315, 37–40 (2015)
Udem, T. et al. Absolute optical frequency measurement of the cesium D1 line with a mode-locked laser. Phys. Rev. Lett. 82, 3568–3571 (1999)
Diddams, S. A. et al. Direct link between microwave and optical frequencies with a 300 THz femtosecond laser comb. Phys. Rev. Lett. 84, 5102–5105 (2000)
Adler, F. et al. Phase-stabilized, 1.5 W frequency comb at 2.8–4.8 μm. Opt. Lett. 34, 1330–1332 (2009)
Brown, G. G. et al. A broadband Fourier transform microwave spectrometer based on chirped pulse excitation. Rev. Sci. Instrum. 79, 053103 (2008)
Park, G. B. et al. Design and evaluation of a pulsed-jet chirped-pulse millimeter-wave spectrometer for the 70–102 GHz region. J. Chem. Phys. 135, 024202 (2011)
Patterson, D. & Doyle, J. M. Cooling molecules in a cell for FTMW spectroscopy. Mol. Phys. 110, 1757–1766 (2012)
Piskorski, J. et al. Cooling, spectroscopy and non-sticking of trans-stilbene and Nile Red. ChemPhysChem 15, 3800–3804 (2014)
Cavagnat, D. & Lespade, L. Internal dynamics contributions to the CH stretching overtone spectra of gaseous nitromethane NO2CH3 . J. Chem. Phys. 106, 7946–7957 (1997)
Davis, S. et al. Jet-cooled molecular radicals in slit supersonic discharges: sub-Doppler infrared studies of methyl radical. J. Chem. Phys. 107, 5661–5675 (1997)
Brumfield, B. E., Stewart, J. T. & McCall, B. J. Extending the limits of rotationally resolved absorption spectroscopy: pyrene. J. Phys. Chem. Lett. 3, 1985–1988 (2012)
Rohart, F. Microwave spectrum of nitromethane internal rotation Hamiltonian in the low barrier case. J. Mol. Spectrosc. 57, 301–311 (1975)
Sørensen, G. O. et al. Microwave spectra of nitromethane and D3-nitromethane. J. Mol. Struct. 97, 77–82 (1983)
Pimentel, G. C. & McClellan, A. L. The infrared spectra of naphthalene crystals, vapor, and solutions. J. Chem. Phys. 20, 270–277 (1952)
Hewett, K. B. et al. High resolution infrared spectroscopy of pyrazine and naphthalene in a molecular beam. J. Chem. Phys. 100, 4077–4086 (1994)
Pirali, O. et al. The far infrared spectrum of naphthalene characterized by high resolution synchrotron FTIR spectroscopy and anharmonic DFT calculations. Phys. Chem. Chem. Phys. 15, 10141–10150 (2013)
Muñoz Caro, G. M. et al. UV-photoprocessing of interstellar ice analogs: detection of hexamethylenetetramine-based species. Astron. Astrophys. 413, 209–216 (2004)
Fleisher, A. J. et al. Mid-infrared time-resolved frequency comb spectroscopy of transient free radicals. J. Phys. Chem. Lett. 5, 2241–2246 (2014)
Thorpe, M. J. & Ye, J. Cavity-enhanced direct frequency comb spectroscopy. Appl. Phys. B 91, 397–414 (2008)
Brubach, J. et al. Performance of the AILES THz-infrared beamline at SOLEIL for high resolution spectroscopy. AIP Conf. Proc. 1214, 81–84 (2010)
Maslowski, P. et al. Surpassing the path-limited resolution of Fourier-transform spectrometry with frequency combs. Phys. Rev. A 93, 021802(R) (2016)
Cox, A. P. & Waring, S. Microwave spectrum and structure of nitromethane. J. Chem. Soc. Faraday Trans. 2 68, 1060–1071 (1972)
Jones, W. J. & Sheppard, N. The gas-phase infrared spectra of nitromethane and methyl boron difluoride; fine structure caused by internal rotation. Proc. R. Soc. Lond. A 304, 135–155 (1968)
McKean, D. C. & Watt, R. A. Vibrational spectra of nitromethanes and the effects of internal rotation. J. Mol. Spectrosc. 61, 184–202 (1976)
Hill, J. R. et al. Infrared, Raman, and coherent anti-Stokes Raman spectroscopy of the hydrogen/deuterium isotopomers of nitromethane. J. Phys. Chem. 95, 3037–3044 (1991)
Gorse, D. et al. Theoretical and spectroscopic study of asymmetric methyl rotor dynamics in gaseous partially deuterated nitromethanes. J. Phys. Chem. 97, 4262–4269 (1993)
Hazra, A., Ghosh, P. & Kshirsagar, R. Fourier transform infrared spectrum and rotational structure of the A-type 917.5 cm−1 band of nitromethane. J. Mol. Spectrosc. 164, 20–26 (1994)
Hazra, A. & Ghosh, P. Assignment of the m = 0 transitions in the ν4 band of nitromethane by the symmetric top approximation method. J. Mol. Spectrosc. 173, 300–302 (1995)
Pal, C. et al. High resolution Fourier transform infrared spectrum and vibration-rotation analysis of the B-type 1584 cm−1 band of nitromethane. J. Mol. Struct. 407, 165–170 (1997)
Halonen, M. et al. Molecular beam infrared spectrum of nitromethane in the region of the first C-H stretching overtone. J. Phys. Chem. A 102, 9124–9128 (1998)
Bunker, P. R. & Jensen, P. Molecular Symmetry and Spectroscopy 2nd edn (NRC Research Press, Ottawa, 1998)
Watson, J. K. G. Vibrational Spectra and Structure Vol. 6, Ch. 1 (ed. Durig, J. ) (Elsevier, 1977)
Townes, C. H. & Schawlow, A. L. Microwave Spectroscopy (Dover, 1975)
Bemish, R. J. et al. Infrared spectroscopy and ab initio potential energy surface for Ne-C2H2 and Ne-C2HD complexes. J. Chem. Phys. 109, 8968–8978 (1998)
Baer, T. & Hase, W. L. Unimolecular Reaction Dynamics (Oxford Univ. Press, 1996)
Bistričić, L., Baranović, G. & Mlinarić-Majerski, K. A vibrational assignment of adamantane and some of its isotopomers. Empirical versus scaled semiempirical force field. Spectrochim. Acta A 51, 1643–1664 (1995)
Jensen, J. O. Vibrational frequencies and structural determination of adamantane. Spectrochim. Acta A 60, 1895–1905 (2004)
Sellers, H., Pulay, P. & Boggs, J. E. Theoretical prediction of vibrational spectra. 2. Force field, spectroscopically refined geometry, and reassignment of the vibrational spectrum of naphthalene. J. Am. Chem. Soc. 107, 6487–6494 (1985)
Mitra, S. S. & Bernstein, H. J. Vibrational spectra of naphthalene-d0, -α-d4, and -d8 molecules. Can. J. Chem. 37, 553–562 (1959)
Ramachandran, G. & Manogaran, S. Vibrational spectra of adamantanes X10H16 and diamantanes X14H20 (X = C, Si, Ge, Sn): a theoretical study. J. Mol. Struct. THEOCHEM 766, 125–135 (2006)
Jenkins, T. & Lewis, J. A Raman study of adamantane (C10H16), diamantane (C14H20) and triamantane (C18H24) between 10 K and room temperatures. Spectrochim. Acta A 36, 259–264 (1980)
Hudson, B. S. et al. Infrared, Raman, and inelastic neutron scattering spectra of dodecahedrane: an I h molecule in T h site symmetry. J. Phys. Chem. A 109, 3418–3424 (2005)
Karpushenkava, L. S., Kabo, G. J. & Bazyleva, A. B. Structure, frequencies of normal vibrations, thermodynamic properties, and strain energies of the cage hydrocarbons CnHn in the ideal-gas state. J. Mol. Struct. THEOCHEM 913, 43–49 (2009)
Szczepanski, J. et al. Electronic and vibrational spectra of matrix isolated anthracene radical cations: experimental and theoretical aspects. J. Chem. Phys. 98, 4494–4511 (1993)
Bakke, A. et al. Condensed aromatics. Part II. The five-parameter approximation of the in-plane force field of molecular vibrations. Z. Naturforsch. C 34a, 579–584 (1979)
Vala, M. et al. Electronic and vibrational spectra of matrix-isolated pyrene radical cations: theoretical and experimental aspects. J. Phys. Chem. 98, 9187–9196 (1994)
Shinohara, H., Yamakita, Y. & Ohno, K. Raman spectra of polycyclic aromatic hydrocarbons. Comparison of calculated Raman intensity distributions with observed spectra for naphthalene, anthracene, pyrene, and perylene. J. Mol. Struct. 442, 221–234 (1998)
Pechukas, P. Comment on “Densities of vibrational states of given symmetry species”. J. Phys. Chem. 88, 828 (1984)
Nesbitt, D. J. & Field, R. W. Vibrational energy flow in highly excited molecules: role of intramolecular vibrational redistribution. J. Phys. Chem. 100, 12735–12756 (1996)
Buckingham, G. T., Chang, C.-H. & Nesbitt, D. J. High-resolution rovibrational spectroscopy of jet-cooled phenyl radical: the ν 19 out-of-phase symmetric CH stretch. J. Phys. Chem. A 117, 10047–10057 (2013)
Acknowledgements
We acknowledge funding from DARPA SCOUT, AFOSR, NIST and NSF-JILA PFC for this research. J.M.D. and D.P. acknowledge funding from the NSF and HQOC. B.S. is supported through an NRC Postdoctoral Fellowship. O.H.H. is partially supported through a Humboldt Fellowship. P.B.C. is supported by the NSF GRFP (award no. DGE1144083). We thank J. Baraban for input and discussion. We thank D. Perry for providing us with G. O. Sørensen’s original nitromethane ground state data.
Author information
Authors and Affiliations
Contributions
P.B.C., D.P., J.M.D. and J.Y. originally designed this experiment. B.S., P.B.C. and J.Y. discussed and implemented the experimental technique, and B.S. and P.B.C. analysed all data. B.S., P.B.C., B.J.B. and O.H.H. operated laboratory equipment. All authors wrote the paper and contributed to technical discussions regarding this work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 Reduced term values of the rotational sub-levels of ν3 + ν6 (m = 0).
These are plotted against the total angular momentum, J (scaled as J(J + 1)). The reduced energies are equal to the absolute energy E, offset by 2,950 cm−1, minus J(J + 1) times the average of the B and C rotational constants. The solid lines connect sets of levels with respect to Ka (the projection of J onto the molecular inertial a axis) and their parity (e/f) symmetry label. For clarity, e and f states are shown in triangles and circles, respectively. States of different Ka values are shown in different colours. Inset, magnified view of the boxed area of the main plot, showing pairs of perturbed eigenstates, split symmetrically about the zeroth-order bright state position, are indicated in bold markers (see Methods for additional details).
Extended Data Figure 2 Evidence of cluster-free cooling.
The plot compares our measured buffer gas cooled C2H2 spectrum (bottom trace) with that of the Ne–C2H2 complex (upper trace; reprinted with permission from figure 1 of ref. 45, copyright 1998, AIP Publishing LLC). Three acetylene monomer transitions in the buffer gas cooled spectrum, including two hot band transitions and a 13C feature as described in the text, have been labelled. The buffer gas cooled spectrum has been rebinned with a bin size of 5 frequency elements (~40 MHz total).
Extended Data Figure 3 The vibrational density of states for several large hydrocarbons.
In increasing order, the total density of states (that is, not symmetry selected) versus vibrational energy is shown for adamantane (C10H16), naphthalene (C10H8), dodecahedrane (C20H20), diamantane (C14H20), anthracene (C14H10), and pyrene (C16H10). These curves were calculated using a direct state count algorithm and a combination of previously observed and calculated vibrational frequencies (see Methods for details). The horizontal line at 100 states per cm−1 marks the empirical threshold symmetry selected state density for IVR60,61. The vertical line at 3,000 cm−1 indicates the approximate energy for CH stretch fundamental vibrations.
Rights and permissions
About this article
Cite this article
Spaun, B., Changala, P., Patterson, D. et al. Continuous probing of cold complex molecules with infrared frequency comb spectroscopy. Nature 533, 517–520 (2016). https://doi.org/10.1038/nature17440
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature17440
This article is cited by
-
Absolute frequency metrology of buffer-gas-cooled molecular spectra at 1 kHz accuracy level
Nature Communications (2022)
-
Dual-comb cavity ring-down spectroscopy
Scientific Reports (2022)
-
High-resolution spectroscopy of buffer-gas-cooled phthalocyanine
Communications Chemistry (2022)
-
Molecular collisions: From near-cold to ultra-cold
Frontiers of Physics (2021)
-
Optical frequency metrology in the bending modes region
Communications Physics (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.