Abstract
Rapid eye movement (REM) sleep is a distinct brain state characterized by activated electroencephalogram and complete skeletal muscle paralysis, and is associated with vivid dreams1,2,3. Transection studies by Jouvet first demonstrated that the brainstem is both necessary and sufficient for REM sleep generation2, and the neural circuits in the pons have since been studied extensively4,5,6,7,8. The medulla also contains neurons that are active during REM sleep9,10,11,12,13, but whether they play a causal role in REM sleep generation remains unclear. Here we show that a GABAergic (γ-aminobutyric-acid-releasing) pathway originating from the ventral medulla powerfully promotes REM sleep in mice. Optogenetic activation of ventral medulla GABAergic neurons rapidly and reliably initiated REM sleep episodes and prolonged their durations, whereas inactivating these neurons had the opposite effects. Optrode recordings from channelrhodopsin-2-tagged ventral medulla GABAergic neurons showed that they were most active during REM sleep (REMmax), and during wakefulness they were preferentially active during eating and grooming. Furthermore, dual retrograde tracing showed that the rostral projections to the pons and midbrain and caudal projections to the spinal cord originate from separate ventral medulla neuron populations. Activating the rostral GABAergic projections was sufficient for both the induction and maintenance of REM sleep, which are probably mediated in part by inhibition of REM-suppressing GABAergic neurons in the ventrolateral periaqueductal grey. These results identify a key component of the pontomedullary network controlling REM sleep. The capability to induce REM sleep on command may offer a powerful tool for investigating its functions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Aserinsky, E. & Kleitman, N. Regularly occurring periods of eye motility, and concomitant phenomena, during sleep. Science 118, 273–274 (1953)
Jouvet, M. Recherches sur les structures nerveuses et les mécanismes responsables des différentes phases du sommeil physiologique. Arch. Ital. Biol. 100, 125–206 (1962)
Dement, W. The occurrence of low voltage, fast, electroencephalogram patterns during behavioral sleep in the cat. Electroencephalogr. Clin. Neurophysiol. 10, 291–296 (1958)
Hobson, J. A., McCarley, R. W. & Wyzinski, P. W. Sleep cycle oscillation: reciprocal discharge by two brainstem neuronal groups. Science 189, 55–58 (1975)
Boissard, R., Fort, P., Gervasoni, D., Barbagli, B. & Luppi, P. H. Localization of the GABAergic and non-GABAergic neurons projecting to the sublaterodorsal nucleus and potentially gating paradoxical sleep onset. Eur. J. Neurosci. 18, 1627–1639 (2003)
Clément, O., Sapin, E., Bérod, A., Fort, P. & Luppi, P. H. Evidence that neurons of the sublaterodorsal tegmental nucleus triggering paradoxical (REM) sleep are glutamatergic. Sleep 34, 419–423 (2011)
Lu, J., Sherman, D., Devor, M. & Saper, C. B. A putative flip-flop switch for control of REM sleep. Nature 441, 589–594 (2006)
Van Dort, C. J. et al. Optogenetic activation of cholinergic neurons in the PPT or LDT induces REM sleep. Proc. Natl Acad. Sci. USA 112, 584–589 (2015)
Siegel, J. M., Wheeler, R. L. & McGinty, D. J. Activity of medullary reticular formation neurons in the unrestrained cat during waking and sleep. Brain Res. 179, 49–60 (1979)
Sakai, K., Kanamori, N. & Jouvet, M. Activités unitaires spécifiques du sommeil paradoxal dans la formation réticulée bulbaire chez le chat non-restreint. C.R. Seances Acad. Sci. D 289, 557–561 (1979)
Maloney, K. J., Mainville, L. & Jones, B. E. c-Fos expression in GABAergic, serotonergic, and other neurons of the pontomedullary reticular formation and raphe after paradoxical sleep deprivation and recovery. J. Neurosci. 20, 4669–4679 (2000)
Sapin, E. et al. Localization of the brainstem GABAergic neurons controlling paradoxical (REM) sleep. PLoS One 4, e4272 (2009)
Sirieix, C., Gervasoni, D., Luppi, P. H. & Léger, L. Role of the lateral paragigantocellular nucleus in the network of paradoxical (REM) sleep: an electrophysiological and anatomical study in the rat. PLoS One 7, e28724 (2012)
Jego, S. et al. Optogenetic identification of a rapid eye movement sleep modulatory circuit in the hypothalamus. Nature Neurosci. 16, 1637–1643 (2013)
Armbruster, B. N., Li, X., Pausch, M. H., Herlitze, S. & Roth, B. L. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc. Natl Acad. Sci. USA 104, 5163–5168 (2007)
Kovács, K. J. c-Fos as a transcription factor: a stressful (re)view from a functional map. Neurochem. Int. 33, 287–297 (1998)
Holmes, C. J. & Jones, B. E. Importance of cholinergic, GABAergic, serotonergic and other neurons in the medial medullary reticular formation for sleep-wake states studied by cytotoxic lesions in the cat. Neuroscience 62, 1179–1200 (1994)
Aston-Jones, G., Ennis, M., Pieribone, V. A., Nickell, W. T. & Shipley, M. T. The brain nucleus locus coeruleus: restricted afferent control of a broad efferent network. Science 234, 734–737 (1986)
Aston-Jones, G. & Bloom, F. E. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J. Neurosci. 1, 876–886 (1981)
Loewy, A. D., Wallach, J. H. & McKellar, S. Efferent connections of the ventral medulla oblongata in the rat. Brain Res. Rev. 3, 63–80 (1981)
Magoun, H. W. & Rhines, R. An inhibitory mechanism in the bulbar reticular formation. J. Neurophysiol. 9, 165–171 (1946)
Schenkel, E. & Siegel, J. M. REM sleep without atonia after lesions of the medial medulla. Neurosci. Lett. 98, 159–165 (1989)
Vanni-Mercier, G., Sakai, K., Lin, J. S. & Jouvet, M. Carbachol microinjections in the mediodorsal pontine tegmentum are unable to induce paradoxical sleep after caudal pontine and prebulbar transections in the cat. Neurosci. Lett. 130, 41–45 (1991)
Webster, H. H., Friedman, L. & Jones, B. E. Modification of paradoxical sleep following transections of the reticular formation at the pontomedullary junction. Sleep 9, 1–23 (1986)
Kohyama, J., Lai, Y. Y. & Siegel, J. M. Inactivation of the pons blocks medullary-induced muscle tone suppression in the decerebrate cat. Sleep 21, 695–699 (1998)
Siegel, J. M., Nienhuis, R. & Tomaszewski, K. S. Rostral brainstem contributes to medullary inhibition of muscle tone. Brain Res. 268, 344–348 (1983)
Carter, M. E. et al. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nature Neurosci. 13, 1526–1533 (2010)
White, S. R., Fung, S. J. & Barnes, C. D. Norepinephrine effects on spinal motoneurons. Prog. Brain Res. 88, 343–350 (1991)
Kaur, S., Saxena, R. N. & Mallick, B. N. GABAergic neurons in prepositus hypoglossi regulate REM sleep by its action on locus coeruleus in freely moving rats. Synapse 42, 141–150 (2001)
Taheri, S., Zeitzer, J. M. & Mignot, E. The role of hypocretins (orexins) in sleep regulation and narcolepsy. Annu. Rev. Neurosci. 25, 283–313 (2002)
Franklin, K.B.J. & Paxinos, G. The Mouse Brain in Stereotaxic Coordinates 3rd edn, 88 (Academic Press, 2007)
Miyamichi, K. et al. Dissecting local circuits: parvalbumin interneurons underlie broad feedback control of olfactory bulb output. Neuron 80, 1232–1245 (2013)
Anikeeva, P. et al. Optetrode: a multichannel readout for optogenetic control in freely moving mice. Nature Neurosci. 15, 163–170 (2012)
Schmitzer-Torbert, N., Jackson, J., Henze, D., Harris, K. & Redish, A. D. Quantitative measures of cluster quality for use in extracellular recordings. Neuroscience 131, 1–11 (2005)
Acknowledgements
We thank A. Popescu for the help with in vivo physiology, M. Bikov and S. Chung for technical assistance, the University of North Carolina Virus Core for supplying AAV, and T. Kilduff and J. Cox for discussions. This work was supported by EMBO and Human Frontier Science Program postdoctoral fellowships (to F.W.).
Author information
Authors and Affiliations
Contributions
F.W. and Y.D. conceived and designed the experiments. F.W. performed all optogenetic stimulation experiments and optrode recordings. S.C. performed a subset of pharmacogenetic experiments and fluorescence microscopy. K.T.B. and L.L. provided viral reagents for rabies-mediated trans-synaptic experiments. M.X. designed the optrodes used in this study. F.W. and Y.D. wrote the manuscript, and all authors participated in the revision of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
All primary histological, electrophysiological, and behavioural data have been archived in the Department of Molecular and Cell Biology, University of California, Berkeley.
Extended data figures and tables
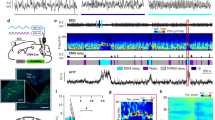
Extended Data Figure 1 Comparison of spontaneous and laser-induced REM sleep.
a, Mean EEG spectrogram and EMG amplitude before and after laser onset (averaged across all trials with laser onset falling on NREM sleep). b, Mean EEG spectrogram and EMG amplitude before and after spontaneous REM onset outside laser stimulation periods (only REM episodes with duration >70 s were included). c, Comparison of EEG power spectra during spontaneous (grey) and laser-induced (blue) REM sleep. Blue shading, s.e.m. for laser-induced REM sleep.
Extended Data Figure 2 Effect of laser stimulation on brain states in eYFP control mice.
a, Brain states in all trials from five mice aligned by time of laser stimulation (top) and probability of wake, NREM, or REM states before, during, and after laser stimulation (bottom). Shading, 95% CI. Blue bar, period of laser stimulation. Laser stimulation caused no significant change in the probability of any brain state (P > 0.34, bootstrap). b, Similar to a, for trials in which laser onset fell on NREM sleep. c, Trials in which laser onset fell on wakefulness.
Extended Data Figure 3 Optogenetic activation of vM glutamatergic neurons induces wakefulness.
a, Probability of wake, NREM, or REM states before, during, and after laser stimulation (20 Hz, 120 s) in VGLUT2-Cre mice injected with AAV expressing ChR2–eYFP into the vM (n = 3 mice). Shading, 95% CI. Blue bar, period of laser stimulation. Laser stimulation caused a significant increase in wakefulness (P < 0.001, bootstrap) and decrease in NREM sleep (P < 0.001). b, Mean durations of REM sleep episodes with and without laser stimulation. Each pair of dots represents data from one mouse. Laser stimulation shortened the duration of REM sleep episodes (n = 3 mice, P = 0.008, paired t-test). Error bar, s.d. c, Probability of wake (left), NREM (middle), and REM (right) states before, during, and after laser stimulation (20 Hz, 120 s) at different laser powers (colour coded).
Extended Data Figure 4 Effect of laser stimulation of vM GABAergic neurons on the transition probability between each pair of brain states in ChR2 and eYFP control mice.
a–f, ChR2 control mice. a, Probability of NREM (N) to REM (R) state transition within each 20 s period in ChR2 mice. Blue shading, period of laser stimulation (20 Hz, 120 s). Error bar, s.d. (bootstrap, n = 6 mice). Probability of baseline transition (red dashed line) was computed after excluding the laser stimulation period. The probability during laser stimulation was significantly higher than the baseline (P = 0.02, bootstrap). b, Similar to a, for NREM to wake (W) transition. The probability during laser stimulation was not significantly different from baseline (P = 0.24). c, REM to wake, probability during laser stimulation was significantly lower than baseline (P < 0.001), consistent with the effect of vM GABAergic neurons on prolonging REM duration (Fig. 2). d, REM to NREM, which rarely occurs in rodents. Laser stimulation caused no significant effect (P > 0.99). e, Wake to REM, which rarely occurs in normal mice. Laser stimulation had no significant effect (P > 0.99). f, Wake to NREM. Laser stimulation caused a significant reduction in the transition probability (P < 0.001), indicating that during wakefulness vM GABAergic neuron activity has a wake-maintenance effect. g–l, Similar to a–f, for eYFP control mice. Laser stimulation had no significant effect on any transition probability (P > 0.05).
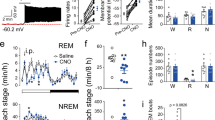
Extended Data Figure 5 Pharmacogenetic inactivation of vM GABAergic neurons reduces REM sleep.
a, Brain states in a control (vehicle injection) and a CNO session from an example mouse. The recording session started 20 min after vehicle or CNO injection. b, Probability of each brain state during the first 2.5 h of the recording session, after injection of vehicle (grey) or two dosages of CNO (different shades of blue). Error bar, s.e.m. (n = 6 mice). *P < 0.05; **P < 0.01; one-way analysis of variance with post hoc Dunnett’s test. c, Similar to b, but during the second half of the recording session (2.5–5 h). There was no significant difference between control and CNO at any dosage (P > 0.12). d, Latency of first REM sleep episode (from the beginning of each recording session). e, Frequency of REM episodes during the first 2.5 h of the recording session. f, Duration of REM episodes during the first 2.5 h of the session. The reduction of REM sleep caused by pharmacogenetic inactivation of vM GABAergic neurons appears to be due to the reduction of frequency rather than duration of REM episodes.
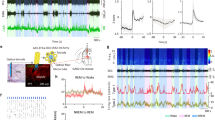
Extended Data Figure 6 Optogenetic identification of vM GABAergic neurons.
a, Left, reliability and temporal jitter of laser-evoked spikes in identified (blue) and unidentified (grey) units recorded in the vM. Note that the identified and unidentified units form two distinct clusters, with high reliability and low jitter for identified units. Dark/light symbols, data during 30/15 Hz laser stimulation. Middle, distribution of delays of laser-evoked spiking for 21 identified GABAergic neurons. Delay is defined as timing of the first spike after each laser pulse. Right, distribution of correlation coefficient between laser-evoked and spontaneous spike waveforms for all 21 identified vM GABAergic neurons. b, Fluorescence image of a coronal section showing the position of an electrolytic lesion at the end of the optrode tract (arrow). Blue, DAPI staining. c, Positions of the 21 identified vM GABAergic neurons from 5 mice. Each dot indicates one neuron. All 20 neurons with recording periods encompassing all three brain states showed maximal firing rates during REM sleep. The wakeful behaviour during which the neuron showed the maximal firing rate is colour-coded. Black, neurons for which the recording period did not include all wakeful behaviours. Schemes of brain sections adapted from Allen Mouse Brain Atlas (Website: © 2015 Allen Institute for Brain Science. Allen Mouse Brain Atlas [Internet]. Available from: http://mouse.brain-map.org).
Extended Data Figure 7 Activity of vM GABAergic neurons at REM sleep onset and offset.
a, Mean firing rates of vM GABAergic neurons at REM onset. Shading, s.e.m. Dark blue, period in which firing rate was significantly higher than baseline (P < 0.05, Wilcoxon signed-rank test); baseline was defined as the average firing rate during the 10 s intervals 60 s before and after REM sleep. b, Mean firing rates at REM offset.
Extended Data Figure 8 Effect of laser stimulation of vM neurons on several wakeful behaviours.
a, Probability of moving, running, eating, and grooming before, during, and after laser stimulation (20 Hz, 120 s) in GAD2-Cre mice injected with AAV expressing ChR2–eYFP into the vM (n = 8 mice). Unclassified behaviours are not shown. Blue bar, period of laser stimulation. Laser stimulation caused a significant increase in eating (P = 0.008, Wilcoxon signed-rank test). b, Similar to a, but in control mice expressing eYFP (n = 4 mice).
Extended Data Figure 9 Firing rates of unidentified vM neurons.
a, Firing rates of unidentified units in the three brain states. Each line represents data from one neuron. Gray bar represents average over units (n = 24). b, Left, relative firing rates of the units across brain states. The firing rates of each unit were normalized by its maximum. Right, normalized firing rates across different wakeful behaviours. c, Mean firing rates of unidentified vM neurons at REM onset. Shading, s.e.m. Dark blue, period in which firing rate was significantly higher than baseline (P < 0.05, Wilcoxon signed-rank test). d, Mean firing rates of unidentified vM neurons at REM offset.
Extended Data Figure 10 Co-expression of GABA and glycine in vM neurons.
a, Fluorescence image of ChR2–eYFP expressing neurons in the vM of a GAD2-Cre mouse injected with Cre-inducible AAV. b, Immunohistochemical staining for glycine. c, Superposition of eYFP expression and glycine staining. In total, 94% (273/289) of eYFP-expressing cells were glycine positive (n = 3 mice).
Supplementary information
Optogenetic activation of vM GABAergic neurons induces REM sleep
The video shows 3 laser stimulation trials, including 1 min before and after each laser stimulation period. In the first two trials the animal was in NREM sleep at laser onset, and he was awake in the third trial. The EEG spectrogram, EMG amplitude and hypnogram are shown on the right. Laser stimulation periods are depicted by the blue shading on the top right and additionally indicated as a blue square in the upper right corner of the movie frame; 10x speedup. (MOV 2003 kb)
Firing rates of an identified vM GABAergic neuron during sleep and wakeful behaviors
The video shows two recording periods of an identified unit. During the first period the animal was asleep, during the second period he was awake and engaged in multiple behaviors (ET – eating, GR – grooming, MV – moving, RU – running, QA – quiet awake). EEG spectrogram, EMG amplitude, hypnogram and firing rate are shown on the right. The time points of single spikes are represented as vertical lines on the bottom left. Laser stimulation periods (15 or 30 Hz) are shown as blue shadings along with the firing rate and are indicated by a blue square within the movie frame; 10x speedup. (MOV 2400 kb)
Rights and permissions
About this article
Cite this article
Weber, F., Chung, S., Beier, K. et al. Control of REM sleep by ventral medulla GABAergic neurons. Nature 526, 435–438 (2015). https://doi.org/10.1038/nature14979
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature14979
This article is cited by
-
Neuro-orchestration of sleep and wakefulness
Nature Neuroscience (2023)
-
A common neuronal ensemble in nucleus accumbens regulates pain-like behaviour and sleep
Nature Communications (2023)
-
Prefrontal cortical regulation of REM sleep
Nature Neuroscience (2023)
-
Sleep fMRI with simultaneous electrophysiology at 9.4 T in male mice
Nature Communications (2023)
-
Neural Control of REM Sleep and Motor Atonia: Current Perspectives
Current Neurology and Neuroscience Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.