Abstract
To sustain neurotransmission, synaptic vesicles and their associated proteins must be recycled locally at synapses. Synaptic vesicles are thought to be regenerated approximately 20 s after fusion by the assembly of clathrin scaffolds or in approximately 1 s by the reversal of fusion pores via ‘kiss-and-run’ endocytosis. Here we use optogenetics to stimulate cultured hippocampal neurons with a single stimulus, rapidly freeze them after fixed intervals and examine the ultrastructure using electron microscopy—‘flash-and-freeze’ electron microscopy. Docked vesicles fuse and collapse into the membrane within 30 ms of the stimulus. Compensatory endocytosis occurs within 50 to 100 ms at sites flanking the active zone. Invagination is blocked by inhibition of actin polymerization, and scission is blocked by inhibiting dynamin. Because intact synaptic vesicles are not recovered, this form of recycling is not compatible with kiss-and-run endocytosis; moreover, it is 200-fold faster than clathrin-mediated endocytosis. It is likely that ‘ultrafast endocytosis’ is specialized to restore the surface area of the membrane rapidly.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Heuser, J. E. & Reese, T. S. Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. J. Cell Biol. 57, 315–344 (1973)
Ceccarelli, B., Hurlbut, W. P. & Mauro, A. Depletion of vesicles from frog neuromuscular junctions by prolonged tetanic stimulation. J. Cell Biol. 54, 30–38 (1972)
Torri-Tarelli, F., Grohovaz, F., Fesce, R. & Ceccarelli, B. Temporal coincidence between synaptic vesicle fusion and quantal secretion of acetylcholine. J. Cell Biol. 101, 1386–1399 (1985)
Zhang, Q., Cao, Y.-Q. & Tsien, R. W. Quantum dots provide an optical signal specific to full collapse fusion of synaptic vesicles. Proc. Natl Acad. Sci. USA 104, 17843–17848 (2007)
Richards, D. A., Bai, J. & Chapman, E. R. Two modes of exocytosis at hippocampal synapses revealed by rate of FM1-43 efflux from individual vesicles. J. Cell Biol. 168, 929–939 (2005)
Von Gersdorff, H. & Matthews, G. Dynamics of synaptic vesicle fusion and membrane retrieval in synaptic terminals. Nature 367, 735–739 (1994)
Dittman, J. & Ryan, T. A. Molecular circuitry of endocytosis at nerve terminals. Annu. Rev. Cell Dev. Biol. 25, 133–160 (2009)
Maycox, P. R., Link, E., Reetz, A., Morris, S. A. & Jahn, R. Clathrin-coated vesicles in nervous tissue are involved primarily in synaptic vesicle recycling. J. Cell Biol. 118, 1379–1388 (1992)
Takei, K. et al. Generation of coated intermediates of clathrin-mediated endocytosis on protein-free liposomes. Cell 94, 131–141 (1998)
Koenig, J. H. & Ikeda, K. Disappearance and reformation of synaptic vesicle membrane upon transmitter release observed under reversible blockage of membrane retrieval. J. Neurosci. 9, 3844–3860 (1989)
Shupliakov, O. et al. Synaptic vesicle endocytosis impaired by disruption of dynamin-SH3 domain interactions. Science 276, 259–263 (1997)
Gu, M. et al. AP2 hemicomplexes contribute independently to synaptic vesicle endocytosis. eLife 2, e00190 (2013)
Kim, S. H. & Ryan, T. A. Synaptic vesicle recycling at CNS snapses without AP-2. J. Neurosci. 29, 3865–3874 (2009)
Nonet, M. L. et al. UNC-11, a Caenorhabditis elegans AP180 homologue, regulates the size and protein composition of synaptic vesicles. Mol. Biol. Cell 10, 2343–2360 (1999)
Diril, M. K., Wienisch, M., Jung, N., Klingauf, J. & Haucke, V. Stonin 2 is an AP-2-dependent endocytic sorting adaptor for synaptotagmin internalization and recycling. Dev. Cell 10, 233–244 (2006)
Schmidt, A. et al. Endophilin I mediates synaptic vesicle formation by transfer of arachidonate to lysophosphatidic acid. Nature 401, 133–141 (1999)
Verstreken, P. et al. Endophilin mutations block clathrin-mediated endocytosis but not neurotransmitter release. Cell 109, 101–112 (2002)
Anggono, V. et al. Syndapin I is the phosphorylation-regulated dynamin I partner in synaptic vesicle endocytosis. Nature Neurosci. 9, 752–760 (2006)
Jakobsson, J. et al. Role of epsin 1 in synaptic vesicle endocytosis. Proc. Natl Acad. Sci. USA 105, 6445–6450 (2008)
Koh, T.-W., Verstreken, P. & Bellen, H. J. Dap160/intersectin acts as a stabilizing scaffold required for synaptic development and vesicle endocytosis. Neuron 43, 193–205 (2004)
Marie, B. et al. Dap160/intersectin scaffolds the periactive zone to achieve high-fidelity endocytosis and normal synaptic growth. Neuron 43, 207–219 (2004)
Cocucci, E., Aguet, F., Boulant, S. & Kirchhausen, T. The first five seconds in the life of a clathrin-coated pit. Cell 150, 495–507 (2012)
Maeno-Hikichi, Y., Polo-Parada, L., Kastanenka, K. V. & Landmesser, L. T. Frequency-dependent modes of synaptic vesicle endocytosis and exocytosis at adult mouse neuromuscular junctions. J. Neurosci. 31, 1093–1105 (2011)
Sato, K. et al. Differential requirements for clathrin in receptor-mediated endocytosis and maintenance of synaptic vesicle pools. Proc. Natl Acad. Sci. USA 106, 1139–1144 (2009)
Teng, H., Cole, J. C., Roberts, R. L. & Wilkinson, R. S. Endocytic active zones: hot spots for endocytosis in vertebrate neuromuscular terminals. J. Neurosci. 19, 4855–4866 (1999)
Harata, N. C., Choi, S., Pyle, J. L., Aravanis, A. M. & Tsien, R. W. Frequency-dependent kinetics and prevalence of kiss-and-run and reuse at hippocampal synapses studied with novel quenching methods. Neuron 49, 243–256 (2006)
Park, H., Li, Y. & Tsien, R. W. Influence of synaptic vesicle position on release probability and exocytotic fusion mode. Science 335, 1362–1366 (2012)
Ryan, T. A., Smith, S. J. & Reuter, H. The timing of synaptic vesicle endocytosis. Proc. Natl Acad. Sci. USA 93, 5567–5571 (1996)
Renden, R. & von Gersdorff, H. Synaptic vesicle endocytosis at a CNS nerve terminal: faster kinetics at physiological temperatures and increased endocytotic capacity during maturation. J. Neurophysiol. 98, 3349–3359 (2007)
Hallermann, S., Pawlu, C., Jonas, P. & Heckmann, M. A large pool of releasable vesicles in a cortical glutamatergic synapse. Proc. Natl Acad. Sci. USA 100, 8975–8980 (2003)
Balaji, J. & Ryan, T. A. Single-vesicle imaging reveals that synaptic vesicle exocytosis and endocytosis are coupled by a single stochastic mode. Proc. Natl Acad. Sci. USA 104, 20576–20581 (2007)
Granseth, B., Odermatt, B., Royle, S. J. & Lagnado, L. Clathrin-mediated endocytosis is the dominant mechanism of vesicle retrieval at hippocampal synapses. Neuron 51, 773–786 (2006)
Miller, T. M. & Heuser, J. E. Endocytosis of synaptic vesicle membrane at the frog neuromuscular junction. J. Cell Biol. 98, 685–698 (1984)
Watanabe, S. et al. Ultrafast endocytosis at Caenorhabditis elegans neuromuscular junctions. eLife 2, e00723 (2013)
Gunaydin, L. A. et al. Ultrafast optogenetic control. Nature Neurosci. 13, 387–392 (2010)
Berndt, A. et al. High-efficiency channelrhodopsins for fast neuronal stimulation at low light levels. Proc. Natl Acad. Sci. USA 108, 7595–7600 (2011)
Pyott, S. J. & Rosenmund, C. The effects of temperature on vesicular supply and release in autaptic cultures of rat and mouse hippocampal neurons. J. Physiol. 539, 523–535 (2002)
Matthews, G. & Sterling, P. Evidence that vesicles undergo compound fusion on the synaptic ribbon. J. Neurosci. 28, 5403–5411 (2008)
He, L. et al. Compound vesicle fusion increases quantal size and potentiates synaptic transmission. Nature 459, 93–97 (2009)
Neale, E. A., Bowers, L. M., Jia, M., Bateman, K. E. & Williamson, L. C. Botulinum neurotoxin A blocks synaptic vesicle exocytosis but not endocytosis at the nerve terminal. J. Cell Biol. 147, 1249–1260 (1999)
Varoqueaux, F. et al. Total arrest of spontaneous and evoked synaptic transmission but normal synaptogenesis in the absence of Munc13-mediated vesicle priming. Proc. Natl Acad. Sci. USA 99, 9037–9042 (2002)
Siksou, L. et al. A common molecular basis for membrane docking and functional priming of synaptic vesicles. Eur. J. Neurosci. 30, 49–56 (2009)
Armbruster, M., Messa, M., Ferguson, S. M., De Camilli, P. & Ryan, T. A. Dynamin phosphorylation controls optimization of endocytosis for brief action potential bursts. eLife 2, e00845 (2013)
Mooren, O. L., Galletta, B. J. & Cooper, J. A. Roles for actin assembly in endocytosis. Annu. Rev. Biochem. 81, 661–686 (2012)
Spector, I., Shochet, N. R., Kashman, Y. & Groweiss, A. Latrunculins: novel marine toxins that disrupt microfilament organization in cultured cells. Science 219, 493–495 (1983)
Macia, E. et al. Dynasore, a cell-permeable inhibitor of dynamin. Dev. Cell 10, 839–850 (2006)
Yamada, H. et al. Dynasore, a dynamin inhibitor, suppresses lamellipodia formation and cancer cell invasion by destabilizing actin filaments. Biochem. Biophys. Res. Commun. 390, 1142–1148 (2009)
McMahon, H. T. & Boucrot, E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nature Rev. Mol. Cell Biol. 12, 517–533 (2011)
Wienisch, M. & Klingauf, J. Vesicular proteins exocytosed and subsequently retrieved by compensatory endocytosis are nonidentical. Nature Neurosci. 9, 1019–1027 (2006)
Lois, C., Hong, E. J., Pease, S., Brown, E. J. & Baltimore, D. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science 295, 868–872 (2002)
Rost, B. R. et al. Autaptic cultures of single hippocampal granule cells of mice and rats. Eur. J. Neurosci. 32, 939–947 (2010)
Studer, D., Humbel, B. M. & Chiquet, M. Electron microscopy of high pressure frozen samples: bridging the gap between cellular ultrastructure and atomic resolution. Histochem. Cell Biol. 130, 877–889 (2008)
Kremer, J. R., Mastronarde, D. N. & McIntosh, J. R. Computer visualization of three-dimensional image data using IMOD. J. Struct. Biol. 116, 71–76 (1996)
Cardona, A. et al. TrakEM2 software for neural circuit reconstruction. PLoS ONE 7, e38011 (2012)
Acknowledgements
We would like to thank D. Lorenz and A. Muenster-Wandowski for providing access to electron microscopes. We would like to thank P. Hegemann and F. Schneider for providing the ChetaTC construct, A. Felies for cell cultures, B. Brokowski for generating lentivirus, E. Hujber for image processing and freezing calculations, C. Ebeling for calculating the time constant for synaptic vesicle collapse, and J. Iwasa for drawing the model figure. We would like to thank C. Tomova and Leica Microsystems for providing us with technical details of the controller of the high-pressure freezer for precise temporal control of light stimulation. We thank EMBO for providing the travel funds (S.W.). The research was funded by the US National Institutes of Health (NS034307; E.M.J.), European Research Council grant (249939 SYNVGLUT; C.R.), and German Research Council grants (EXC 257, SFB 665, SFB958; C.R.). E.M.J. is an Investigator of the Howard Hughes Medical Institute and is an Alexander von Humboldt Scholar.
Author information
Authors and Affiliations
Contributions
S.W., C.R., and E.M.J. conceived and designed experiments. M.W.D. designed and programmed the light device. S.W. and B.S.-K. performed the freezing experiments. B.R.R. designed lentivirus constructs. B.R.R. and M.C.-P. performed electrophysiology. S.W. performed electron microscopy imaging and analysis. S.W., B.R.R., M.C.-P., C.R. and E.M.J. wrote the manuscript. C.R. and E.M.J. provided funding, experiments were performed at the Charité Universitätsmedizin, Berlin, Germany.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 A schematic summarizing ultrafast endocytosis.
Exocytosis in the active zone is followed by rapid internalization of membrane at the edge of the active zone. Synaptic vesicles directly in contact with plasma membrane fuse about 2 ms after an action potential and collapse into membrane. Ultrafast endocytosis occurs at the edge of active zone within 100 ms after stimulation and is mediated by actin and dynamin. The endocytic structures are larger than synaptic vesicles. AZ, active zone; PSD, postsynaptic density.
Extended Data Figure 2 Channelrhodopsin induces action-potential-driven vesicle fusion.
a, Cell-attached voltage clamp recordings of single light-evoked action potentials in presence of synaptic blockers NBQX and bicuculline. b, Number of action potentials triggered during the 10 ms light pulse. Most cells fired at least one action potential during the light pulse (88%, 30/34), though some cells did not respond to the light stimulus (12%, 4/34). Some cells fired a second action potential (26%, 7/34). c, A histogram showing the average number of action potentials at different time points before and after the light application. Each column is binned by 10 ms. Action potentials observed after light-off are likely due to the spontaneous activity of cells. d, Freezing protocol for 15 ms samples. A single light pulse was fired at 0 ms. At 7 ms after light onset, the chamber was flooded with liquid nitrogen. At 8 ms after application of liquid nitrogen, the sample should reach 0 °C (when the chamber temperature is at −20 °C), so that the sample was frozen 15 ms after light onset. Action potentials are initiated 4.8 ms after light onset on average. Thus, the 15 ms freeze is capturing events that occurred on average 10.2 ms after the action potential.
Extended Data Figure 3 Light and electrical stimulation in the same cell elicit identical postsynaptic currents.
a, Paired whole-cell recordings of ChetaTC-expressing (YFP) and non-expressing neurons that were co-cultured on small microislands of astrocytes. Action potentials were triggered every 10 s in the ChetaTC-positive cell by alternating between 10 ms current injection via the patch pipette and 10 ms light flashes. b, Example of a cell pair with a GABAergic ChetaTC-positive neuron. Presynaptic action potentials were recorded in current clamp (CC) of the ChetaTC-positive cell. Postsynaptic inhibitory postsynaptic currents (IPSCs) were recorded in voltage clamp (VC) from the non-infected neuron (bottom panel; holding potential −50 mV). c, Presynaptic action potentials and excitatory postsynaptic currents (EPSCs) evoked by light or somatic depolarization in a pair of neurons with a glutamatergic ChetaTC-positive neuron. The postsynaptic cell was voltage-clamped at −70 mV. d, Time plot of postsynaptic responses triggered by alternating light-induced depolarization (blue bars/open symbols) and electrical stimulation (current step/closed symbols). Both EPSC and IPSC amplitudes were normalized to the first response evoked by electrical stimulation and pooled (n = 9). e, Scatter plot of postsynaptic currents evoked by ChetaTC stimulation normalized to direct electrical stimulation in the same neuron. Reponses are not different for the two modes of action potential induction (P = 0.77, one sample t-test). Error bars, s.e.m.
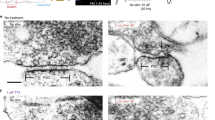
Extended Data Figure 4 Large invaginations adjacent to the active zone are endocytic intermediates.
a–l, Additional representative micrographs showing shallow invaginations 50 ms after stimulation (a, e, i), deep invaginations 100 ms after stimulation (b, c, f, g, j, k) and large vesicles at the edge of active zone 300 ms after stimulation (d, h, l). m, o, Coats are usually absent on ultrafast invaginations, but can occasionally be observed (j, k, arrow). n, p, But these differ from those observed at classical clathrin coated pits (n) or vesicles (p). The coated pit (n) was not observed at the synapse but was rather at the plasma membrane of soma.
Extended Data Figure 5 Large invaginations take up a fluid phase marker.
a–e, Additional representative micrographs showing ferritin uptake in the whole terminal in non-stimulation control (a) and 100 ms after stimulation (b) and by large structures at the edge of active zones 100 ms after stimulation (c–e). An arrow in b indicates a ferritin-positive shallow pit. Ferritin was applied to cells for 5 min before the light stimulation. Note that very little ferritin was internalized during the pre-incubation period, suggesting that the ferritin distribution is associated acutely with endocytosis after the stimulation. In unstimulated cultures, 18% of the profiles exhibited some internalization of ferritin; of those profiles only 1 vesicle from the ∼56 total vesicles contained ferritin. At 100 ms, 26% of the profiles exhibited synaptic vesicles with ferritin (P = 0.29); of those profiles that had internalized ferritin only 1 vesicle from 51.2 total vesicles contained ferritin. Thus, ultrafast endocytosis did not directly generate detectable numbers of synaptic vesicles with ferritin. f, Number of shallow pits (<40 nm), deep pits (>40 nm), large vesicles associated with the membrane (<5 nm) and large vesicles associated with the plasma membrane (6–50 nm of the membrane) in controls and ferritin-containing seeded cultures. In ferritin seeded cultures the large vesicles near the membrane (within 5 nm) all contained ferritin. The total number of ferritin-positive endocytic structures was 0.38 ± 0.03 endocytic structures per profile, similar to the endocytosis values obtained in Fig. 3i without ferritin (0.43 ± 0.03 endocytic structures per profile). g, Diameter of large vesicles (86.0 ± 2.4; n = 51) and endocytic invaginations (77.3 ± 3 nm; n = 16; P = 0.33). ‘No ferritin’ controls are from the samples in Fig. 3. Error bars, s.e.m.
Extended Data Figure 6 Ultrafast endocytosis occurs with similar dynamics in 2 mM and 4 mM extracellular calcium.
a, A histogram showing the average number of action potentials at different time points before and after the light application in an external solution with 2 mM calcium. Each column is binned by 10 ms. Action potentials observed after light-off are probably due to spontaneous activity of cells. b, Average number of action potentials triggered during the 10 ms light pulse. An action potential was observed during the light pulse in 73% of the cells (11/15); 13% (2/15) fired a second action potential within the 10 ms light pulse. No action potential was observed in 27% of the cells (4/15). c–e, Representative micrographs showing invaginations and large vesicles in the periactive zone at 100 ms after stimulation. f, g, Average number of exocytic pits (f) and endocytic structures (g) at 0 ms (no stim), 30 ms and 100 ms after stimulation in 2 mM calcium (light grey) and in 4 mM calcium (dark grey) conditions. Endocytic structures include shallow and deep pits summed. 4 mM data are from the samples in Figs 1 and 3. Error bars, s.e.m.
Extended Data Figure 7 Ultrafast endocytosis is mediated by actin.
a, Electrophysiological recordings on mixed cultures. PSCs were recorded from ChetaTC-negative neurons (red fluorescence) while stimulating ChetaTC/YFP-positive neurons with light. b, Scatter plot of postsynaptic currents evoked by channelrhodopsin stimulation in 10 µM latrunculin-A/0.1% DMSO normalized to postsynaptic currents in 0.1% DMSO in the same neuron. 30 s incubation with latrunculin-A has no effect on evoked neurotransmission. Average PSC amplitudes were normalized to average PSC amplitudes preceding latrunculin-A application (n = 8, normalized PSC in latrunculin-A is 0.95 ± 0.08, P = 0.6, one sample t-test). c–h, Additional representative micrographs showing 10 µM latrunculin-A-treated (c–e) and 0.1% DMSO-treated (f–h) cells 100 ms after stimulation. Ultrafast endocytosis occurs in DMSO-treated cells but is completely blocked in latrunculin-A treated cells. Error bar, s.e.m.
Extended Data Figure 8 Ultrafast endocytosis is mediated by dynamin.
a–f, Additional representative micrographs showing 80 µM dynasore-treated cells from 40-nm thick sections (a–c) and a 200-nm tomogram (d–f). Ultrafast endocytic structures are trapped on the surface in the dynasore-treated cells. Note that large vesicles are found associated with the membrane (a–c, d, f) but the necks of the trapped structures are only visible at their centre (a, e) due to the thickness of the sections. Electron tomography was performed on 21 sections (200-nm thick). 9 out of 21 tomograms showed a trapped structure, and all of these trapped structures were connected to the membrane at their centre.
Extended Data Figure 9 Ice crystal damage was not observed in cell cultures subjected to high pressure freezing.
a–f, Sample micrographs show a nucleus (a, d), a mitochondrion (b, e), and a synapse (c, f). a–c, In these samples there is no visible damage by ice crystals. d–f, Samples exhibiting ice crystal damage caused by brief accidental removal from liquid nitrogen. Note the reticulated appearance caused by protein aggregation. Ice damage was not observed in the samples used in this study.
Supplementary information
Supplementary Data
This file contains Supplementary Table 1. (PDF 319 kb)
Rights and permissions
About this article
Cite this article
Watanabe, S., Rost, B., Camacho-Pérez, M. et al. Ultrafast endocytosis at mouse hippocampal synapses. Nature 504, 242–247 (2013). https://doi.org/10.1038/nature12809
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature12809
This article is cited by
-
Mechanisms of SNARE proteins in membrane fusion
Nature Reviews Molecular Cell Biology (2024)
-
Membrane transformations of fusion and budding
Nature Communications (2024)
-
Membrane compression by synaptic vesicle exocytosis triggers ultrafast endocytosis
Nature Communications (2023)
-
Transmission of NLRP3-IL-1β Signals in Cerebral Ischemia and Reperfusion Injury: from Microglia to Adjacent Neuron and Endothelial Cells via IL-1β/IL-1R1/TRAF6
Molecular Neurobiology (2023)
-
Molecular mechanics underlying flat-to-round membrane budding in live secretory cells
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.