Abstract
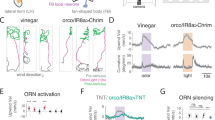
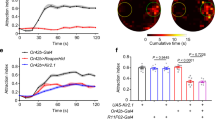
Behavioural responses to wind are thought to have a critical role in controlling the dispersal and population genetics of wild Drosophila species1,2, as well as their navigation in flight3, but their underlying neurobiological basis is unknown. We show that Drosophila melanogaster, like wild-caught Drosophila strains4, exhibits robust wind-induced suppression of locomotion in response to air currents delivered at speeds normally encountered in nature1,2. Here we identify wind-sensitive neurons in Johnston’s organ, an antennal mechanosensory structure previously implicated in near-field sound detection (reviewed in refs 5 and 6). Using enhancer trap lines targeted to different subsets of Johnston’s organ neurons7, and a genetically encoded calcium indicator8, we show that wind and near-field sound (courtship song) activate distinct populations of Johnston’s organ neurons, which project to different regions of the antennal and mechanosensory motor centre in the central brain. Selective genetic ablation of wind-sensitive Johnston’s organ neurons in the antenna abolishes wind-induced suppression of locomotion behaviour, without impairing hearing. Moreover, different neuronal subsets within the wind-sensitive population respond to different directions of arista deflection caused by air flow and project to different regions of the antennal and mechanosensory motor centre, providing a rudimentary map of wind direction in the brain. Importantly, sound- and wind-sensitive Johnston’s organ neurons exhibit different intrinsic response properties: the former are phasically activated by small, bi-directional, displacements of the aristae, whereas the latter are tonically activated by unidirectional, static deflections of larger magnitude. These different intrinsic properties are well suited to the detection of oscillatory pulses of near-field sound and laminar air flow, respectively. These data identify wind-sensitive neurons in Johnston’s organ, a structure that has been primarily associated with hearing, and reveal how the brain can distinguish different types of air particle movements using a common sensory organ.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Johnston, J. & Templeton, A. in Ecological Genetics and Evolution (eds Barker, J. S. F. & Starmer, W. T.) 241–256 (Academic, 1982)
Johnston, J. & Heed, W. Dispersal of desert-adapted Drosophila: the saguaro-breeding D. nigrospiracula. Am. Nat. 110, 629–651 (1976)
Budick, S. A., Reiser, M. B. & Dickinson, M. H. The role of visual and mechanosensory cues in structuring forward flight in Drosophila melanogaster. J. Exp. Biol. 210, 4092–4103 (2007)
Richardson, R. & Johnston, J. Behavioral components of dispersal in Drosophila mimica. Oecologia 20, 287–299 (1975)
Caldwell, J. C. & Eberl, D. F. Towards a molecular understanding of Drosophila hearing. J. Neurobiol. 53, 172–189 (2002)
Kernan, M. J. Mechanotransduction and auditory transduction in Drosophila. Pflügers Arch. 454, 703–720 (2007)
Kamikouchi, A., Shimada, T. & Ito, K. Comprehensive classification of the auditory sensory projections in the brain of the fruit fly Drosophila melanogaster. J. Comp. Neurol. 499, 317–356 (2006)
Nakai, J., Ohkura, M. & Imoto, K. A high signal-to-noise Ca2+ probe composed of a single green fluorescent protein. Nature Biotechnol. 19, 137–141 (2001)
Göpfert, M. C. & Robert, D. The mechanical basis of Drosophila audition. J. Exp. Biol. 205, 1199–1208 (2002)
Mamiya, A. et al. Neural representations of airflow in Drosophila mushroom body. PLoS ONE 3, e4063 (2008)
Manning, A. Antennae and sexual receptivity in Drosophila melanogaster females. Science 158, 136–137 (1967)
Kim, J. et al. A TRPV family ion channel required for hearing in Drosophila. Nature 424, 81–84 (2003)
Wang, S. L. et al. The Drosophila caspase inhibitor DIAP1 is essential for cell survival and is negatively regulated by HID. Cell 98, 453–463 (1999)
Eberl, D. F., Hardy, R. W. & Kernan, M. J. Genetically similar transduction mechanisms for touch and hearing in Drosophila. J. Neurosci. 20, 5981–5988 (2000)
Tanouye, M. A. & Wyman, R. J. Motor outputs of giant nerve fiber in Drosophila. J. Neurophysiol. 44, 405–421 (1980)
Bennet-Clark, H. C. Acoustics of insect song. Nature 234, 255–259 (1971)
Moffat, K. G. et al. Inducible cell ablation in Drosophila by cold-sensitive ricin A chain. Development 114, 681–687 (1992)
Wong, A. M., Wang, J. W. & Axel, R. Spatial representation of the glomerular map in the Drosophila protocerebrum. Cell 109, 229–241 (2002)
Ewing, A. W. The antenna of Drosophila as a ‘love song’ receptor. Physiol. Entomol. 3, 33–36 (1978)
Ikeda, I. et al. Selective phototoxic destruction of rat Merkel cells abolishes responses of slowly adapting type I mechanoreceptor units. J. Physiol. (Lond.) 479, 247–256 (1994)
Hoffmann, J. N., Montag, A. G. & Dominy, N. J. Meissner corpuscles and somatosensory acuity: the prehensile appendages of primates and elephants. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 281, 1138–1147 (2004)
Kamikouchi, A. et al. The neural basis of Drosophila gravity-sensing and hearing. Nature 1010.1038/nature07810 (this issue)
Wang, J. W., Wong, A. M., Flores, J., Vosshall, L. B. & Axel, R. Two-photon calcium imaging reveals an odor-evoked map of activity in the fly brain. Cell 112, 271–282 (2003)
Wang, Y. et al. Stereotyped odor-evoked activity in the mushroom body of Drosophila revealed by green fluorescent protein-based Ca2+ imaging. J. Neurosci. 24, 6507–6514 (2004)
Berdnik, D., Chihara, T., Couto, A. & Luo, L. Wiring stability of the adult Drosophila olfactory circuit after lesion. J. Neurosci. 26, 3367–3376 (2006)
Wheeler, D. A., Fields, W. L. & Hall, J. C. Spectral analysis of Drosophila courtship songs: D. melanogaster, D. simulans, and their interspecific hybrid. Behav. Genet. 18, 675–703 (1988)
Acknowledgements
We thank U. Heberlein and F. Wolf for hosting a sabbatical that led to the discovery of WISL; J. S. Johnson for helpful discussions; L. Zelnik, M. Reiser and P. Perona for creating locomotor tracking software; D. Eberl and J. Hall for D. melanogaster courtship song recordings; G. Maimon for making fly holders for imaging experiments; M. Roy for building behavioral chambers for WISL and female receptivity assays; H. Inagaki for JO-CE-GAL4;eyFLP flies; B. Hay for UAS-hid flies; D. Berdnik for UAS-FRT-STOP-FRT-Ricin flies; M. Dickinson for anemometers and discussions; J. L. Anderson for advice on fluid mechanics; M. Göpfert for providing a pressure gradient microphone; M. Konishi for advice and use of laboratory facilities; and G. Mosconi for laboratory management. D.J.A. is an Investigator of the Howard Hughes Medical Institute. This work was supported in part by NSF grant EF-0623527.
Author Contributions S.Y. and D.J.A. designed experiments, S.Y. carried out all experiments reported in this paper and D.J.A. and S.Y. wrote the manuscript. A.W. wrote Matlab programs for ΔF/F measurements and mechanical probe actuation, B.J.F. assisted with computational filtering of song stimuli, H.D. assisted with computational and statistical analysis of data, M.J.K. provided facilities and support for electrophysiological experiments, and A.K. and K.I. provided Gal4 lines.
Author information
Authors and Affiliations
Corresponding authors
Supplementary information
Supplementary Information
This file contains Supplementary Figures S1-S4, Supplementary Notes S1-S4 and Supplementary Movie Legends. (PDF 3074 kb)
Supplementary Movie 1
This movie shows WISL behavior in Drosophila melanogaster (see file s1 for full legend). (MOV 877 kb)
Supplementary Movie 2a
This movie shows the responses of JO neurons to sound and wind stimuli in zones A, C and E, using the Gal4 line JO-ACE to drive expression of UAS-GCaMP (see file s1 for full legend). (MOV 1218 kb)
Supplementary Movie 2b
This movie shows the responses of JO neurons to sound and wind stimuli in zones A, C and E, using the Gal4 line JO-ACE to drive expression of UAS-GCaMP (see file s1 for full legend). (MOV 1228 kb)
Supplementary Movie 2c
This movie shows the responses of JO neurons to sound and wind stimuli in zones A, C and E, using the Gal4 line JO-ACE to drive expression of UAS-GCaMP (see file s1 for full legend). (MOV 1258 kb)
Supplementary Movie 2d
This movie shows the responses of JO neurons to sound and wind stimuli in zones A, C and E, using the Gal4 line JO-ACE to drive expression of UAS-GCaMP (see file s1 for full legend). (MOV 1252 kb)
Supplementary Movie 2e
This movie shows the responses of JO neurons to sound and wind stimuli in zones A, C and E, using the Gal4 line JO-ACE to drive expression of UAS-GCaMP (see file s1 for full legend). (MOV 1200 kb)
Supplementary Movie 3a
This movie shows the responses of zone C and E neurons to wind delivered from different directions, using the Gal4 line JO-CE to drive expression of GCaMP (see file s1 for full legend). (MOV 2694 kb)
Supplementary Movie 3b
This movie shows the responses of zone C and E neurons to wind delivered from different directions, using the Gal4 line JO-CE to drive expression of GCaMP (see file s1 for full legend). (MOV 2623 kb)
Supplementary Movie 3c
This movie shows the responses of zone C and E neurons to wind delivered from different directions, using the Gal4 line JO-CE to drive expression of GCaMP (see file s1 for full legend). (MOV 2727 kb)
Supplementary Movie 3d
This movie shows the responses of zone C and E neurons to wind delivered from different directions, using the Gal4 line JO-CE to drive expression of GCaMP (see file s1 for full legend). (MOV 2746 kb)
Supplementary Movie 3e
This movie shows the responses of zone C and E neurons to wind delivered from different directions, using the Gal4 line JO-CE to drive expression of GCaMP (see file s1 for full legend). (MOV 2668 kb)
Supplementary Movie 4a
This movie was made to show the direction of aristae deflection during the presentation of wind stimuli, under the same conditions used to obtain the data illustrated in Supplementary Movie 3 (see file s1 for full legend). (MOV 440 kb)
Supplementary Movie 4b
This movie was made to show the direction of aristae deflection during the presentation of wind stimuli, under the same conditions used to obtain the data illustrated in Supplementary Movie 3 (see file s1 for full legend). (MOV 415 kb)
Supplementary Movie 4c
This movie was made to show the direction of aristae deflection during the presentation of wind stimuli, under the same conditions used to obtain the data illustrated in Supplementary Movie 3 (see file s1 for full legend). (MOV 427 kb)
Supplementary Movie 4d
This movie was made to show the direction of aristae deflection during the presentation of wind stimuli, under the same conditions used to obtain the data illustrated in Supplementary Movie 3 (see file s1 for full legend). (MOV 446 kb)
Supplementary Movie 4e
This movie was made to show the direction of aristae deflection during the presentation of wind stimuli, under the same conditions used to obtain the data illustrated in Supplementary Movie 3 (see file s1 for full legend). (MOV 429 kb)
Rights and permissions
About this article
Cite this article
Yorozu, S., Wong, A., Fischer, B. et al. Distinct sensory representations of wind and near-field sound in the Drosophila brain. Nature 458, 201–205 (2009). https://doi.org/10.1038/nature07843
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature07843
This article is cited by
-
Threat gates visual aversion via theta activity in Tachykinergic neurons
Nature Communications (2023)
-
Evolutionary conservation and diversification of auditory neural circuits that process courtship songs in Drosophila
Scientific Reports (2023)
-
Olfactory navigation in arthropods
Journal of Comparative Physiology A (2023)
-
Wing Modulation and Aerodynamics of Hoverflies in Gust Perturbations
Journal of Bionic Engineering (2023)
-
Acetylcholine deficit causes dysfunctional inhibitory control in an aging-dependent manner
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.