Abstract
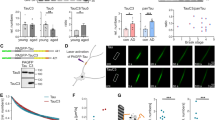
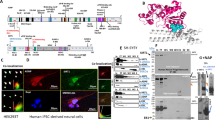
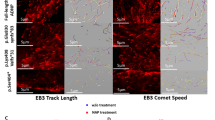
Activity-dependent neuroprotective protein (ADNP), vital for brain formation and cognitive function, is mutated in autism and linked to neurodegenerative/psychiatric diseases. An eight-amino-acid peptide snippet of ADNP, NAP (NAPVSIPQ), identified as a smallest active fragment, includes the SxIP microtubule (MT) end-binding protein (EB) association motif, and enhances ADNP–EB3 interaction. Depletion of EB1 or EB3 abolishes NAP protection against zinc intoxication. Furthermore, NAP enhances Tau–MT interaction, and Tau regulates the localization and function of EB1 and EB3 in developing neuronal cells. Here, we asked how NAP (ADNP) enhances Tau–MT interactions and whether this is mediated by EBs. We showed, for we believe the first time, that NAP augmented endogenous EB1 comet density in the N1E-115 neuroblastoma neuronal model. This finding was substantiated by cell transfection with fluorescent EB1 and live cell imaging. NAP increased comet amounts, length and speed. At the molecular level, NAP enhanced EB3 homodimer formation, while decreasing EB1–EB3 heterodimer content and driving EB1– and EB3–Tau interactions (dramatic 20-fold increases), leading to recruitment of EB1/EB3 and Tau to MTs under zinc intoxication. Our previous results showed that while NAP protected neuronal-like cells against oxidative stress, it did not protect NIH3T3 fibroblasts. Here, NAP did not protect NIH3T3 cells against zinc intoxication, unless these cells were transfected with Tau. Interestingly, other MT associated proteins (MAPs) may replace Tau, thus, EB–Tau (MAPs) interaction is identified as a novel target for endogenous ADNP neuroprotection, and a future target for drug development, with NAP as a prototype.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Mandel S, Rechavi G, Gozes I . Activity-dependent neuroprotective protein (ADNP) differentially interacts with chromatin to regulate genes essential for embryogenesis. Dev Biol 2007; 303: 814–824.
Pinhasov A, Mandel S, Torchinsky A, Giladi E, Pittel Z, Goldsweig AM et al. Activity-dependent neuroprotective protein: a novel gene essential for brain formation. Brain Res Dev Brain Res 2003; 144: 83–90.
Mandel S, Spivak-Pohis I, Gozes I . ADNP differential nucleus/cytoplasm localization in neurons suggests multiple roles in neuronal differentiation and maintenance. J Mol Neurosci 2008; 35: 127–141.
Amram N, Hacohen-Kleiman G, Sragovich S, Malishkevich A, Katz J, Touloumi O et al. Sexual divergence in microtubule function: the novel intranasal microtubule targeting SKIP normalizes axonal transport and enhances memory. Mol Psychiatry 2016; 21: 1467–1476.
Oz S, Kapitansky O, Ivashco-Pachima Y, Malishkevich A, Giladi E, Skalka N et al. The NAP motif of activity-dependent neuroprotective protein (ADNP) regulates dendritic spines through microtubule end binding proteins. Mol Psychiatry 2014; 19: 1115–1124.
Pascual M, Guerri C . The peptide NAP promotes neuronal growth and differentiation through extracellular signal-regulated protein kinase and Akt pathways, and protects neurons co-cultured with astrocytes damaged by ethanol. J Neurochem 2007; 103: 557–568.
Vulih-Shultzman I, Pinhasov A, Mandel S, Grigoriadis N, Touloumi O, Pittel Z et al. Activity-dependent neuroprotective protein snippet NAP reduces tau hyperphosphorylation and enhances learning in a novel transgenic mouse model. J Pharmacol Exp Ther 2007; 323: 438–449.
Malishkevich A, Leyk J, Goldbaum O, Richter-Landsberg C, Gozes I . ADNP/ADNP2 expression in oligodendrocytes: implication for myelin-related neurodevelopment. J Mol Neurosci 2015; 57: 304–313.
Helsmoortel C, Vulto-van Silfhout AT, Coe BP, Vandeweyer G, Rooms L, van den Ende J et al. A SWI/SNF-related autism syndrome caused by de novo mutations in ADNP. Nat Genet 2014; 46: 380–384.
Gozes I, Helsmoortel C, Vandeweyer G, Van der Aa N, Kooy F, Sermone SB . The compassionate side of neuroscience: Tony Sermone's undiagnosed genetic journey—ADNP mutation. J Mol Neurosci 2015; 56: 751–757.
Malishkevich A, Marshall GA, Schultz AP, Sperling RA, Aharon-Peretz J, Gozes I . Blood-borne activity-dependent neuroprotective protein (ADNP) is correlated with premorbid intelligence, clinical stage, and Alzheimer's disease biomarkers. J Alzheimers Dis 2015; 50: 249–260.
Chu Y, Morfini GA, Kordower JH . Alterations in activity-dependent neuroprotective protein in sporadic and experimental Parkinson's disease. J Parkinsons Dis 2016; 6: 77–97.
Merenlender-Wagner A, Malishkevich A, Shemer Z, Udawela M, Gibbons A, Scarr E et al. Autophagy has a key role in the pathophysiology of schizophrenia. Mol Psychiatry 2015; 20: 126–132.
Mandel S, Gozes I . Activity-dependent neuroprotective protein constitutes a novel element in the SWI/SNF chromatin remodeling complex. J Biol Chem 2007; 282: 34448–34456.
Batsche E, Yaniv M, Muchardt C . The human SWI/SNF subunit Brm is a regulator of alternative splicing. Nat Struct Mol Biol 2006; 13: 22–29.
Furman S, Steingart RA, Mandel S, Hauser JM, Brenneman DE, Gozes I . Subcellular localization and secretion of activity-dependent neuroprotective protein in astrocytes. Neuron Glia Biol 2004; 1: 193–199.
Schirer Y, Malishkevich A, Ophir Y, Lewis J, Giladi E, Gozes I . Novel marker for the onset of frontotemporal dementia: early increase in activity-dependent neuroprotective protein (ADNP) in the face of Tau mutation. PLoS One 2014; 9: e87383.
Gozes I, Divinski I, Piltzer I . NAP and D-SAL: neuroprotection against the beta amyloid peptide (1-42). BMC Neurosci 2008; 9 (Suppl 3): S3.
Bassan M, Zamostiano R, Davidson A, Pinhasov A, Giladi E, Perl O et al. Complete sequence of a novel protein containing a femtomolar-activity-dependent neuroprotective peptide. J Neurochem 1999; 72: 1283–1293.
Divinski I, Mittelman L, Gozes I . A femtomolar acting octapeptide interacts with tubulin and protects astrocytes against zinc intoxication. J Biol Chem 2004; 279: 28531–28538.
Smith-Swintosky VL, Gozes I, Brenneman DE, D'Andrea MR, Plata-Salaman CR . Activity-dependent neurotrophic factor-9 and NAP promote neurite outgrowth in rat hippocampal and cortical cultures. J Mol Neurosci 2005; 25: 225–238.
Gozes I, Divinski I . NAP, a neuroprotective drug candidate in clinical trials, stimulates microtubule assembly in the living cell. Curr Alzheimer Res 2007; 4: 507–509.
Jouroukhin Y, Ostritsky R, Assaf Y, Pelled G, Giladi E, Gozes I . NAP (davunetide) modifies disease progression in a mouse model of severe neurodegeneration: protection against impairments in axonal transport. Neurobiol Dis 2013; 56: 79–94.
Oz S, Ivashko-Pachima Y, Gozes I . The ADNP derived peptide, NAP modulates the tubulin pool: implication for neurotrophic and neuroprotective activities. PLoS One 2012; 7: e51458.
Sayas CL, Tortosa E, Bollati F, Ramirez-Rios S, Arnal I, Avila J . Tau regulates the localization and function of End-binding proteins 1 and 3 in developing neuronal cells. J Neurochem 2015; 133: 653–667.
Kimhi Y, Palfrey C, Spector I, Barak Y, Littauer UZ . Maturation of neuroblastoma cells in the presence of dimethylsulfoxide. Proc Natl Acad Sci USA 1976; 73: 462–466.
Aronov S, Aranda G, Behar L, Ginzburg I . Axonal tau mRNA localization coincides with tau protein in living neuronal cells and depends on axonal targeting signal. J Neurosci 2001; 21: 6577–6587.
Bulinski JC, Gruber D, Faire K, Prasad P, Chang W . GFP chimeras of E-MAP-115 (ensconsin) domains mimic behavior of the endogenous protein in vitro and in vivo. Cell Struct Funct 1999; 24: 313–320.
Malishkevich A, Amram N, Hacohen-Kleiman G, Magen I, Giladi E, Gozes I . Activity-dependent neuroprotective protein (ADNP) exhibits striking sexual dichotomy impacting on autistic and Alzheimer's pathologies. Transl Psychiatry 2015; 5: e501.
Hu X, Pickering E, Liu YC, Hall S, Fournier H, Katz E et al. Meta-analysis for genome-wide association study identifies multiple variants at the BIN1 locus associated with late-onset Alzheimer's disease. PLoS One 2011; 6: e16616.
Lotjonen J, Wolz R, Koikkalainen J, Julkunen V, Thurfjell L, Lundqvist R et al. Fast and robust extraction of hippocampus from MR images for diagnostics of Alzheimer's disease. Neuroimage 2011; 56: 185–196.
Komarova Y, De Groot CO, Grigoriev I, Gouveia SM, Munteanu EL, Schober JM et al. Mammalian end binding proteins control persistent microtubule growth. J Cell Biol 2009; 184: 691–706.
Divinski I, Holtser-Cochav M, Vulih-Schultzman I, Steingart RA, Gozes I . Peptide neuroprotection through specific interaction with brain tubulin. J Neurochem 2006; 98: 973–984.
Lee G, Newman ST, Gard DL, Band H, Panchamoorthy G . Tau interacts with src-family non-receptor tyrosine kinases. J Cell Sci 1998; 111 (Pt 21): 3167–3177.
Lee G, Rook SL . Expression of tau protein in non-neuronal cells: microtubule binding and stabilization. J Cell Sci 1992; 102 (Pt 2): 227–237.
Varleta L, Salas O, Toledo P, Iglesias J, Tisne L, Dabancens A et al. Cervical smears in the study of intraepithelial neoplasms. Rev Chil Obstet Ginecol 1978; 43: 344–351.
Barlan K, Lu W, Gelfand VI . The microtubule-binding protein ensconsin is an essential cofactor of kinesin-1. Curr Biol 2013; 23: 317–322.
De Groot CO, Jelesarov I, Damberger FF, Bjelic S, Scharer MA, Bhavesh NS et al. Molecular insights into mammalian end-binding protein heterodimerization. J Biol Chem 2010; 285: 5802–5814.
Sen I, Veprintsev D, Akhmanova A, Steinmetz MO . End binding proteins are obligatory dimers. PLoS One 2013; 8: e74448.
Jaworski J, Kapitein LC, Gouveia SM, Dortland BR, Wulf PS, Grigoriev I et al. Dynamic microtubules regulate dendritic spine morphology and synaptic plasticity. Neuron 2009; 61: 85–100.
Bjelic S, De Groot CO, Scharer MA, Jaussi R, Bargsten K, Salzmann M et al. Interaction of mammalian end binding proteins with CAP-Gly domains of CLIP-170 and p150(glued). J Struct Biol 2012; 177: 160–167.
Moughamian AJ, Osborn GE, Lazarus JE, Maday S, Holzbaur EL . Ordered recruitment of dynactin to the microtubule plus-end is required for efficient initiation of retrograde axonal transport. J Neurosci 2013; 33: 13190–13203.
Yenjerla M, LaPointe NE, Lopus M, Cox C, Jordan MA, Feinstein SC et al. The neuroprotective peptide NAP does not directly affect polymerization or dynamics of reconstituted neural microtubules. J Alzheimers Dis 2010; 19: 1377–1386.
Benbow SJ, Cook BM, Reifert J, Wozniak KM, Slusher BS, Littlefield BA et al. Effects of paclitaxel and eribulin in mouse sciatic nerve: a microtubule-based rationale for the differential induction of chemotherapy-induced peripheral neuropathy. Neurotox Res 2016; 29: 299–313.
Jarskog LF, Dong Z, Kangarlu A, Colibazzi T, Girgis RR, Kegeles LS et al. Effects of davunetide on N-acetylaspartate and choline in dorsolateral prefrontal cortex in patients with schizophrenia. Neuropsychopharmacology 2013; 38: 1245–1252.
Gozes I, Stewart A, Morimoto B, Fox A, Sutherland K, Schmeche D . Addressing Alzheimer's disease tangles: from NAP to AL-108. Curr Alzheimer Res 2009; 6: 455–460.
Morimoto BH, Schmechel D, Hirman J, Blackwell A, Keith J, Gold M et al. A double-blind, placebo-controlled, ascending-dose, randomized study to evaluate the safety, tolerability and effects on cognition of AL-108 after 12 weeks of intranasal administration in subjects with mild cognitive impairment. Dement Geriatr Cogn Disord 2013; 35: 325–336.
Boxer AL, Lang AE, Grossman M, Knopman DS, Miller BL, Schneider LS et al. Davunetide in patients with progressive supranuclear palsy: a randomised, double-blind, placebo-controlled phase 2/3 trial. Lancet Neurol 2014; 13: 676–685.
Gozes I, Ivashko-Pachima Y . ADNP: in search for molecular mechanisms and innovative therapeutic strategies for frontotemporal degeneration. Front Aging Neurosci 2015; 7: 205.
Quraishe S, Cowan CM, Mudher A . NAP (davunetide) rescues neuronal dysfunction in a Drosophila model of tauopathy. Mol Psychiatry 2013; 18: 834–842.
Matsuoka Y, Gray AJ, Hirata-Fukae C, Minami SS, Waterhouse EG, Mattson MP et al. Intranasal NAP administration reduces accumulation of amyloid peptide and tau hyperphosphorylation in a transgenic mouse model of Alzheimer's disease at early pathological stage. J Mol Neurosci 2007; 31: 165–170.
Matsuoka Y, Jouroukhin Y, Gray AJ, Ma L, Hirata-Fukae C, Li HF et al. A neuronal microtubule-interacting agent, NAPVSIPQ, reduces tau pathology and enhances cognitive function in a mouse model of Alzheimer's disease. J Pharmacol Exp Ther 2008; 325: 146–153.
Shiryaev N, Jouroukhin Y, Giladi E, Polyzoidou E, Grigoriadis NC, Rosenmann H et al. NAP protects memory, increases soluble tau and reduces tau hyperphosphorylation in a tauopathy model. Neurobiol Dis 2009; 34: 381–388.
Sudo H, Baas PW . Strategies for diminishing katanin-based loss of microtubules in tauopathic neurodegenerative diseases. Hum Mol Genet 2011; 20: 763–778.
Tortosa E, Galjart N, Avila J, Sayas CL . MAP1B regulates microtubule dynamics by sequestering EB1/3 in the cytosol of developing neuronal cells. EMBO J 2013; 32: 1293–1306.
Ramirez-Rios S, Denarier E, Prezel E, Vinit A, Stoppin-Mellet V, Devred F et al. Tau antagonizes EB tracking at microtubule ends through a phosphorylation-dependent mechanism. Mol Biol Cell 2016; 27: 2924–2934.
Magen I, Ostritsky R, Richter F, Zhu C, Fleming SM, Lemesre V et al. Intranasal NAP (davunetide) decreases tau hyperphosphorylation and moderately improves behavioral deficits in mice overexpressing alpha-synuclein. Pharmacol Res Persp 2014; 2: e00065.
Dresner E, Agam G, Gozes I . Activity-dependent neuroprotective protein (ADNP) expression level is correlated with the expression of the sister protein ADNP2: deregulation in schizophrenia. Eur Neuropsychopharmacol 2011; 21: 355–361.
Liu X, Bipolar Genome Study Kelsoe JR, Greenwood TA . A genome-wide association study of bipolar disorder with comorbid eating disorder replicates the SOX2-OT region. J Affect Disord 2016; 189: 141–149.
Merenlender-Wagner A, Shemer Z, Touloumi O, Lagoudaki R, Giladi E, Andrieux A et al. New horizons in schizophrenia treatment: autophagy protection is coupled with behavioral improvements in a mouse model of schizophrenia. Autophagy 2014; 10: 2324–2332.
Vaisburd S, Shemer Z, Yeheskel A, Giladi E, Gozes I . Risperidone and NAP protect cognition and normalize gene expression in a schizophrenia mouse model. Sci Rep 2015; 5: 16300.
Javitt DC, Buchanan RW, Keefe RS, Kern R, McMahon RP, Green MF et al. Effect of the neuroprotective peptide davunetide (AL-108) on cognition and functional capacity in schizophrenia. Schizophr Res 2012; 136: 25–31.
Kumar P, Wittmann T . +TIPs: SxIPping along microtubule ends. Trends Cell Biol 2012; 22: 418–428.
Akhmanova A, Steinmetz MO . Control of microtubule organization and dynamics: two ends in the limelight. Nat Revi Mol Cell Biol 2015; 16: 711–726.
Acknowledgements
We are grateful to Drs Stella Aronov, Department of Molecular Biology, Ariel University, Ariel, Israel; Doron Chabat, Raymond and Beverly Sackler Faculty of Exact Sciences, Tel Aviv University, Tel Aviv, Israel and Eran Perlson, Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel, for providing valuable reagents, including the Tau plasmid, the labeled NAP and the EMTB plasmid, respectively. Professor Gozes Laboratory is supported in part by the AMN Foundation, Israel Science Foundation, ERA-NET-NEURON, the Dr. Diana and Zelman Elton (Elbaum) Laboratory for Molecular Neuroendocrinology and the Lily and Avraham Gildor Chair for the Investigation of Growth Factors at Tel Aviv University. YI-P is supported by a Joseph Sagol PhD fellowship in brain studies, in the Dr. Miriam and Sheldon Adelson Graduate School at the Sackler Faculty of Medicine, Tel Aviv University. CLS was supported by the IMBRAIN project (FP7-REGPOT-2012-CT2012–31637-IMBRAIN), funded under the 7th Framework Programme (Capacities).
Author contributions
YI-P designed and performed the work; CLS provided the neuroblastoma cell line and in-depth knowhow for EB imaging; AM provided knowhow for immunoprecipitations and IG supervised the work and guided research plans and manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
NAP is under patent protection and term sheet agreement (Ramot at the Tel Aviv University and Coronis Neurosciences, Israel; IG, the inventor and Chief Scientific Officer). The remaining authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Supplementary information
Rights and permissions
About this article
Cite this article
Ivashko-Pachima, Y., Sayas, C., Malishkevich, A. et al. ADNP/NAP dramatically increase microtubule end-binding protein–Tau interaction: a novel avenue for protection against tauopathy. Mol Psychiatry 22, 1335–1344 (2017). https://doi.org/10.1038/mp.2016.255
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2016.255
This article is cited by
-
Clinical impact and in vitro characterization of ADNP variants in pediatric patients
Molecular Autism (2024)
-
ADNP dysregulates methylation and mitochondrial gene expression in the cerebellum of a Helsmoortel–Van der Aa syndrome autopsy case
Acta Neuropathologica Communications (2024)
-
Loss-of-function of activity-dependent neuroprotective protein (ADNP) by a splice-acceptor site mutation causes Helsmoortel–Van der Aa syndrome
European Journal of Human Genetics (2024)
-
Chromatin remodeler Activity-Dependent Neuroprotective Protein (ADNP) contributes to syndromic autism
Clinical Epigenetics (2023)
-
The cytoplasmic localization of ADNP through 14-3-3 promotes sex-dependent neuronal morphogenesis, cortical connectivity, and calcium signaling
Molecular Psychiatry (2023)