Abstract
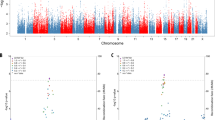
The genetic basis of Alzheimer's disease (AD) is complex and heterogeneous. Over 200 highly penetrant pathogenic variants in the genes APP, PSEN1, and PSEN2 cause a subset of early-onset familial AD. On the other hand, susceptibility to late-onset forms of AD (LOAD) is indisputably associated to the ɛ4 allele in the gene APOE, and more recently to variants in more than two-dozen additional genes identified in the large-scale genome-wide association studies (GWAS) and meta-analyses reports. Taken together however, although the heritability in AD is estimated to be as high as 80%, a large proportion of the underlying genetic factors still remain to be elucidated. In this study, we performed a systematic family-based genome-wide association and meta-analysis on close to 15 million imputed variants from three large collections of AD families (~3500 subjects from 1070 families). Using a multivariate phenotype combining affection status and onset age, meta-analysis of the association results revealed three single nucleotide polymorphisms (SNPs) that achieved genome-wide significance for association with AD risk: rs7609954 in the gene PTPRG (P-value=3.98 × 10−8), rs1347297 in the gene OSBPL6 (P-value=4.53 × 10−8), and rs1513625 near PDCL3 (P-value=4.28 × 10−8). In addition, rs72953347 in OSBPL6 (P-value=6.36 × 10−7) and two SNPs in the gene CDKAL1 showed marginally significant association with LOAD (rs10456232, P-value=4.76 × 10−7; rs62400067, P-value=3.54 × 10−7). In summary, family-based GWAS meta-analysis of imputed SNPs revealed novel genomic variants in (or near) PTPRG, OSBPL6, and PDCL3 that influence risk for AD with genome-wide significance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Canadian study of health and aging: study methods and prevalence of dementia. CMAJ 1994; 150: 899–913.
Cure Alzheimer's Fund. 2015; Available from http://curealz.org/alzheimers-disease.
Alzheimer's Assoc. Facts & Figures. 2015; Available from http://www.alz.org/facts/overview.asp.
Glenner GG, Wong CW . Alzheimer's disease and Down's syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun 1984; 122: 1131–1135.
Gatz M, Reynolds CA, Fratiglioni L, Johansson B, Mortimer JA, Berg S et al. Role of genes and environments for explaining Alzheimer disease. Arch Gen Psychiatry 2006; 63: 168–174.
Laird NM, Horvath S, Xu X . Implementing a unified approach to family-based tests of association. Genet Epidemiol 2000; 19: S36–S42.
Lange C, Silverman EK, Xu X, Weiss ST, Laird NM . A multivariate family-based association test using generalized estimating equations: FBAT-GEE. Biostatistics 2003; 4: 195–206.
Blacker D, Haines JL, Rodes L, Terwedow H, Go RC, Harrell LE et al. ApoE-4 and age at onset of Alzheimer's disease: the NIMH genetics initiative. Neurology 1997; 48: 139–147.
Ku CS, Pawitan Y, Sim X, Ong RT, Seielstad M, Lee EJ et al. Genomic copy number variations in three Southeast Asian populations. Hum Mutat 2010; 31: 851–857.
de Andrade M, Atkinson EJ, Bamlet WR, Matsumoto ME, Maharjan S, Slager SL et al. Evaluating the influence of quality control decisions and software algorithms on SNP calling for the affymetrix 6.0 SNP array platform. Hum Hered 2011; 71: 221–233.
Lee JH, Cheng R, Graff-Radford N, Foroud T, Mayeux R . National Institute on Aging Late-Onset Alzheimer's Disease Family Study G. Analyses of the National Institute on Aging Late-Onset Alzheimer's Disease Family Study: implication of additional loci. Arch Neurol 2008; 65: 1518–1526.
Bertram L, Lange C, Mullin K, Parkinson M, Hsiao M, Hogan MF et al. Genome-wide association analysis reveals putative Alzheimer's disease susceptibility loci in addition to APOE. Am J Hum Genet 2008; 83: 623–632.
Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 2007; 81: 559–575.
Howie BN, Donnelly P, Marchini J . A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet 2009; 5: e1000529.
1,000 Genome Project Reference data. 2013.
Won S, Wilk JB, Mathias RA, O'Donnell CJ, Silverman EK, Barnes K et al. On the analysis of genome-wide association studies in family-based designs: a universal, robust analysis approach and an application to four genome-wide association studies. PLoS Genet 2009; 5: e1000741.
Van Steen K, McQueen MB, Herbert A, Raby B, Lyon H, Demeo DL et al. Genomic screening and replication using the same data set in family-based association testing. Nat Genet 2005; 37: 683–691.
Laird NM, Lange C . Family-based methods for linkage and association analysis. Adv Genet 2008; 60: 219–252.
Lange C, DeMeo D, Silverman EK, Weiss ST, Laird NM . PBAT: tools for family-based association studies. Am J Hum Genet 2004; 74: 367–369.
Magi R, Asimit JL, Day-Williams AG, Zeggini E, Morris AP . Genome-wide association analysis of imputed rare variants: application to seven common complex diseases. Genet Epidemiol 2012; 36: 785–796.
Willer CJ, Li Y, Abecasis GR . METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics 2010; 26: 2190–2191.
Trynka G, Sandor C, Han B, Xu H, Stranger BE, Liu XS et al. Chromatin marks identify critical cell types for fine mapping complex trait variants. Nat Genet 2013; 45: 124–130.
Bertram L, Tanzi RE . Genome-wide association studies in Alzheimer's disease. Hum Mol Genet 2009; 18: R137–R145.
Lambert JC, Ibrahim-Verbaas CA, Harold D, Naj AC, Sims R, Bellenguez C et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer's disease. Nat Genet 2013; 45: 1452–1458.
Reddy JV, Ganley IG, Pfeffer SR . Clues to neuro-degeneration in Niemann-Pick type C disease from global gene expression profiling. PloS One 2006; 1: e19.
Yeger-Lotem E, Riva L, Su LJ, Gitler AD, Cashikar AG, King OD et al. Bridging high-throughput genetic and transcriptional data reveals cellular responses to alpha-synuclein toxicity. Nat Genet 2009; 41: 316–323.
Berchtold NC, Coleman PD, Cribbs DH, Rogers J, Gillen DL, Cotman CW . Synaptic genes are extensively downregulated across multiple brain regions in normal human aging and Alzheimer's disease. Neurobiol Aging 2013; 34: 1653–1661.
Olkkonen VM, Li S . Oxysterol-binding proteins: sterol and phosphoinositide sensors coordinating transport, signaling and metabolism. Prog Lipid Res 2013; 52: 529–538.
Srinivasan S, Meyer RD, Lugo R, Rahimi N . Identification of PDCL3 as a novel chaperone protein involved in the generation of functional VEGF receptor 2. J Biol Chem 2013; 288: 23171–23181.
Wilkinson JC, Richter BW, Wilkinson AS, Burstein E, Rumble JM, Balliu B et al. VIAF, a conserved inhibitor of apoptosis (IAP)-interacting factor that modulates caspase activation. J Biol Chem 2004; 279: 51091–51099.
Baumann K, Mandelkow EM, Biernat J, Piwnica-Worms H, Mandelkow E . Abnormal Alzheimer-like phosphorylation of tau-protein by cyclin-dependent kinases cdk2 and cdk5. FEBS Lett 1993; 336: 417–424.
Li A, Meyre D . Challenges in reproducibility of genetic association studies: lessons learned from the obesity field. Int J Obes 2013; 37: 559–567.
Lange EM, Sun J, Lange LA, Zheng SL, Duggan D, Carpten JD et al. Family-based samples can play an important role in genetic association studies. Cancer Epidemiol Biomarkers Prev 2008; 17: 2208–2214.
Ott J, Kamatani Y, Lathrop M . Family-based designs for genome-wide association studies. Nat Rev Genet. 2011; 12: 465–474.
Evangelou E, Trikalinos TA, Salanti G, Ioannidis JP . Family-based versus unrelated case-control designs for genetic associations. PLoS Genet 2006; 2: e123.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Author contributions
Herold performed pre-GWAS QC, the imputation of the GWAS datasets, statistical analysis of family-based cohorts and drafted the manuscript. Hooli processed DNA samples on human microarray, SNP genotype data generation and quality control, and drafted manuscript. Mullin performed genotyping, data generation and quality assessment. Liu assisted in the imputation of the GWAS datasets, statistical analysis of case-control datasets. Roehr performed the imputation of the various GWAS datasets. Mattheissen performed pre-GWAS QC, statistical analysis and revised the manuscript for intellectual content. Parrado performed pre-GWAS QC and statistical analysis. Bertram helped design, conceptualization, and planning of the study, and manuscript revision. Lange designed, conceptualized, planned, and oversaw the study, and helped with the meta-analysis of the GWAS studies, the GWAS approaches and plan the study. Tanzi designed, conceptualized, planned, and oversaw the study, and helped in drafting and revising the manuscript.
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Rights and permissions
About this article
Cite this article
Herold, C., Hooli, B., Mullin, K. et al. Family-based association analyses of imputed genotypes reveal genome-wide significant association of Alzheimer’s disease with OSBPL6, PTPRG, and PDCL3. Mol Psychiatry 21, 1608–1612 (2016). https://doi.org/10.1038/mp.2015.218
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2015.218
This article is cited by
-
A maximum flow-based network approach for identification of stable noncoding biomarkers associated with the multigenic neurological condition, autism
BioData Mining (2021)
-
Cell-type-specific expression quantitative trait loci associated with Alzheimer disease in blood and brain tissue
Translational Psychiatry (2021)
-
Human Hsp90 cochaperones: perspectives on tissue-specific expression and identification of cochaperones with similar in vivo functions
Cell Stress and Chaperones (2021)
-
Genome-wide DNA methylation analysis of heavy cannabis exposure in a New Zealand longitudinal cohort
Translational Psychiatry (2020)
-
A telescope GWAS analysis strategy, based on SNPs-genes-pathways ensamble and on multivariate algorithms, to characterize late onset Alzheimer’s disease
Scientific Reports (2020)