Abstract
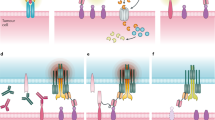
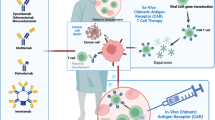
Recent advances in antibody technology to harness T cells for cancer immunotherapy, particularly in the difficult-to-treat setting of relapsed/refractory acute lymphoblastic leukemia (r/r ALL), have led to innovative methods for directing cytotoxic T cells to specific surface antigens on cancer cells. One approach involves administration of soluble bispecific (or dual-affinity) antibody-based constructs that temporarily bridge T cells and cancer cells. Another approach infuses ex vivo-engineered T cells that express a surface plasma membrane-inserted antibody construct called a chimeric antigen receptor (CAR). Both bispecific antibodies and CARs circumvent natural target cell recognition by creating a physical connection between cytotoxic T cells and target cancer cells to activate a cytolysis signaling pathway; this connection allows essentially all cytotoxic T cells in a patient to be engaged because typical tumor cell resistance mechanisms (such as T-cell receptor specificity, antigen processing and presentation, and major histocompatibility complex context) are bypassed. Both the bispecific T-cell engager (BiTE) antibody construct blinatumomab and CD19-CARs are immunotherapies that have yielded encouraging remission rates in CD19-positive r/r ALL, suggesting that they might serve as definitive treatments or bridging therapies to allogeneic hematopoietic cell transplantation. With the introduction of these immunotherapies, new challenges arise related to unique toxicities and distinctive pathways of resistance. An increasing body of knowledge is being accumulated on how to predict, prevent, and manage such toxicities, which will help to better stratify patient risk and tailor treatments to minimize severe adverse events. A deeper understanding of the precise mechanisms of action and immune resistance, interaction with other novel agents in potential combinations, and optimization in the manufacturing process will help to advance immunotherapy outcomes in the r/r ALL setting.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Hoffmann P, Hofmeister R, Brischwein K, Brandl C, Crommer S, Bargou R et al. Serial killing of tumor cells by cytotoxic T cells redirected with a CD19-/CD3-bispecific single-chain antibody construct. Int J Cancer 2005; 115: 98–104.
Nagorsen D, Kufer P, Baeuerle PA, Bargou R . Blinatumomab: a historical perspective. Pharmacol Ther 2012; 136: 334–342.
Gruen M, Bommert K, Bargou RC . T-cell-mediated lysis of B cells induced by a CD19xCD3 bispecific single-chain antibody is perforin dependent and death receptor independent. Cancer Immunol Immunother 2004; 53: 625–632.
Haas C, Krinner E, Brischwein K, Hoffmann P, Lutterbuse R, Schlereth B et al. Mode of cytotoxic action of T cell-engaging BiTE antibody MT110. Immunobiology 2009; 214: 441–453.
Topp MS, Gokbuget N, Stein AS, Zugmaier G, O'Brien S, Bargou RC et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol 2015; 16: 57–66.
Topp MS, Kufer P, Gokbuget N, Goebeler M, Klinger M, Neumann S et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol 2011; 29: 2493–2498.
Klinger M, Brandl C, Zugmaier G, Hijazi Y, Bargou RC, Topp MS et al. Immunopharmacologic response of patients with B-lineage acute lymphoblastic leukemia to continuous infusion of T cell-engaging CD19/CD3-bispecific BiTE antibody blinatumomab. Blood 2012; 119: 6226–6233.
Zhu M, Kratzer A, Johnson J, Holland C, Brandl C, Singh I et al. Pharmacokinetics/pharmacodynamics (PKPD) of blinatumomab in patients with relapsed/refractory B-precursor acute lymphoblastic leukemia (r/r ALL). J Clin Oncol 2015; 33: Abstract 2561.
Zugmaier G, Topp MS, Alekar S, Viardot A, Horst HA, Neumann S et al. Long-term follow-up of serum immunoglobulin levels in blinatumomab-treated patients with minimal residual disease-positive B-precursor acute lymphoblastic leukemia. Blood Cancer J 2014; 4: 244.
von Stackelberg A, Locatelli F, Zugmaier G, Handgretinger R, Trippett TM, Rizzari C et al. Phase I/phase II study of blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. J Clin Oncol 2016; 34: 4381–4389.
Bruggemann M, Raff T, Flohr T, Gokbuget N, Nakao M, Droese J et al. Clinical significance of minimal residual disease quantification in adult patients with standard-risk acute lymphoblastic leukemia. Blood 2006; 107: 1116–1123.
Raff T, Gokbuget N, Luschen S, Reutzel R, Ritgen M, Irmer S et al. Molecular relapse in adult standard-risk ALL patients detected by data from the GMALL 06/99 and 07/03 trials prospective MRD monitoring during and after maintenance treatment:. Blood 2007; 109: 910–915.
Gokbuget N, Kneba M, Raff T, Trautmann H, Bartram CR, Arnold R et al. Adult patients with acute lymphoblastic leukemia and molecular failure display a poor prognosis and are candidates for stem cell transplantation and targeted therapies. Blood 2012; 120: 1868–1876.
Bader P, Kreyenberg H, Henze GHR, Eckert C, Reising M, Willasch A et al. Prognostic value of minimal residual disease quantification before allogeneic stem-cell transplantation in relapsed childhood acute lymphoblastic leukemia: the ALL-REZ BFM study group. J Clin Oncol 2009; 27: 377–384.
Topp MS, Gokbuget N, Zugmaier G, Degenhard E, Goebeler ME, Klinger M et al. Long-term follow-up of hematologic relapse-free survival in a phase 2 study of blinatumomab in patients with MRD in B-lineage ALL. Blood 2012; 120: 5185–5187.
Goekbuget N, Dombret H, Bonifacio M, Reichle A, Graux C, Havelange V et al. BLAST: a confirmatory, single-arm, phase 2 study of blinatumomab, a bispecific T-cell engager (BiTE® antibody construct, in patients with minimal residual disease B-precursor acute lymphoblastic leukemia (ALL). Blood 2014; 124: Abstract 379.
Gökbuget N, Dombret H, Bonifacio M, Reichle A, Graux C, Faul C et al. Long-term outcomes after blinatumomab treatment: follow-up of a phase 2 study in patients (Pts) with minimal residual disease (MRD) positive B-cell precursor acute lymphoblastic leukemia (ALL). Blood 2015; 126: Abstract 680.
Topp MS, Gokbuget N, Zugmaier G, Klappers P, Stelljes M, Neumann S et al. Phase II trial of the anti-CD19 bispecific T cell-engager blinatumomab shows hematologic and molecular remissions in patients with relapsed or refractory B-precursor acute lymphoblastic leukemia. J Clin Oncol 2014; 32: 4134–4140.
Zugmaier G, Gokbuget N, Klinger M, Viardot A, Stelljes M, Neumann S et al. Long-term survival and T-cell kinetics in relapsed/refractory ALL patients who achieved MRD response after blinatumomab treatment. Blood 2015; 126: 2578–2584.
Topp M, Stein A, Gokbuget N, Fielding A, Schuh A, Ribera Santasusana J et al. Blinatumomab improved overall survival in patients with relapsed or refractory philadelphia negative B-cell precursor acute lymphoblastic leukemia in a randomized, open-label phase 3 study (TOWER). Haematologica 2016; 101: 24–25, Abstract S149.
Martinelli G, Dombret H, Chevallier P, Ottmann OG, Goekbuget N, Topp MS et al. Complete molecular and hematologic response in adult patients with relapsed/refractory (R/R) Philadelphia chromosome-positive B-precursor acute lymphoblastic leukemia (ALL) following treatment with blinatumomab: results from a phase 2 single-arm, multicenter study (ALCANTARA). Blood 2015; 126: Abstract 679.
Kantarjian HM, Stein AS, Bargou RC, Grande Garcia C, Larson RA, Stelljes M et al. Blinatumomab treatment of older adults with relapsed/refractory B-precursor acute lymphoblastic leukemia: results from 2 phase 2 studies. Cancer 2016; 122: 2178–2185.
Stein AS, Topp MS, Kantarjian HM, Goekbuget N, Bargou RC, Litzow MR et al. Treatment with anti-CD19 BiTE® blinatumomab in adult patients with relapsed/refractory B-precursor acute lymphoblastic leukemia (r/r ALL) post-allogeneic hematopoietic stem cell transplantation. Blood 2015; 126: Abstract 861.
Stein AS, Topp MS, Gokbuget N, Bargou RC, Dombret H, Larson RA et al. Outcomes of hematopoietic stem cell transplantation (HSCT) among adults with relapsed/refractory (r/r) acute lymphoblastic leukemia (ALL) achieving remission with blinatumomab. Biol Blood Marrow Transplant 2016; 22: S35–S36, Abstract 7134.
Duell J, Dittrich M, Bedke T, Mueller T, Rasche L, Dandekar T et al. Crucial role of regulatory T cells in predicting the outcome of the T cell engaging antibody blinatumomab in relapsed and refractory B precursor ALL patients. Blood 2014; 124: Abstract 2291.
Topp MS, Stelljes M, Zugmaier G, Barnette P, Heffner LT, Trippett TM et al. Re-exposure to blinatumomab after CD19-positive relapse: experience from three trials in patients (pts) with relapsed/refractory B-precursor acute lymphoblastic leukemia (R/R ALL). J Clin Oncol 2015; 33: Abstract 7051.
Kohnke T, Krupka C, Tischer J, Knosel T, Subklewe M . Increase of PD-L1 expressing B-precursor ALL cells in a patient resistant to the CD19/CD3-bispecific T cell engager antibody blinatumomab. J Hematol Oncol 2015; 8: 111.
Brudno JN, Somerville RP, Shi V, Rose JJ, Halverson DC, Fowler DH et al. Allogeneic T cells that express an anti-CD19 chimeric antigen receptor induce remissions of B-cell malignancies that progress after allogeneic hematopoietic stem-cell transplantation without causing graft-versus-host disease. J Clin Oncol 2016; 34: 1112–1121.
Braig F, Brandt A, Goebeler M, Tony HP, Kurze AK, Nollau P et al. Resistance to anti-CD19/CD3 BiTE in acute lymphoblastic leukemia may be mediated by disrupted CD19 membrane trafficking. Blood 2017; 129: 100–104.
Goebeler ME, Knop S, Viardot A, Kufer P, Topp MS, Einsele H et al. Bispecific T-Cell Engager (BiTE) antibody construct blinatumomab for the treatment of patients with relapsed/refractory non-Hodgkin lymphoma: final results from a phase I study. J Clin Oncol 2016; 34: 1104–1111.
Oren R, Hod-Marco M, Haus-Cohen M, Thomas S, Blat D, Duvshani N et al. Functional comparison of engineered T cells carrying a native TCR versus TCR-like antibody-based chimeric antigen receptors indicates affinity/avidity thresholds. J Immunol 2014; 193: 5733–5743.
Schmid DA, Irving MB, Posevitz V, Hebeisen M, Posevitz-Fejfar A, Sarria JC et al. Evidence for a TCR affinity threshold delimiting maximal CD8 T cell function. J Immunol 2010; 184: 4936–4946.
Hudecek M, Lupo-Stanghellini MT, Kosasih PL, Sommermeyer D, Jensen MC, Rader C et al. Receptor affinity and extracellular domain modifications affect tumor recognition by ROR1-specific chimeric antigen receptor T cells. Clin Cancer Res 2013; 19: 3153–3164.
Kandalaft LE, Powell Jr DJ, Coukos G . A phase I clinical trial of adoptive transfer of folate receptor-alpha redirected autologous T cells for recurrent ovarian cancer. J Transl Med 2012; 10: 157.
Hombach AA, Schildgen V, Heuser C, Finnern R, Gilham DE, Abken H . T cell activation by antibody-like immunoreceptors: the position of the binding epitope within the target molecule determines the efficiency of activation of redirected T cells. J Immunol 2007; 178: 4650–4657.
Guest RD, Hawkins RE, Kirillova N, Cheadle EJ, Arnold J, O'Neill A et al. The role of extracellular spacer regions in the optimal design of chimeric immune receptors: evaluation of four different scFvs and antigens. J Immunother 2005; 28: 203–211.
James SE, Greenberg PD, Jensen MC, Lin Y, Wang J, Till BG et al. Antigen sensitivity of CD22-specific chimeric TCR is modulated by target epitope distance from the cell membrane. J Immunol 2008; 180: 7028–7038.
Hudecek M, Sommermeyer D, Kosasih PL, Silva-Benedict A, Liu L, Rader C et al. The nonsignaling extracellular spacer domain of chimeric antigen receptors is decisive for in vivo antitumor activity. Cancer Immunol Res 2015; 3: 125–135.
Kebriaei P, Singh H, Huls MH, Figliola MJ, Bassett R, Olivares S et al. Phase I trials using Sleeping Beauty to generate CD19-specific CAR T cells. J Clin Invest 2016; 126: 3363–3376.
Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med 2014; 6: 224ra225.
Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 2015; 385: 517–528.
Scholler J, Brady TL, Binder-Scholl G, Hwang WT, Plesa G, Hege KM et al. Decade-long safety and function of retroviral-modified chimeric antigen receptor T cells. Sci Transl Med 2012; 4: 132ra153.
Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 2014; 371: 1507–1517.
Gattinoni L, Finkelstein SE, Klebanoff CA, Antony PA, Palmer DC, Spiess PJ et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med 2005; 202: 907–912.
Wrzesinski C, Paulos CM, Kaiser A, Muranski P, Palmer DC, Gattinoni L et al. Increased intensity lymphodepletion enhances tumor treatment efficacy of adoptively transferred tumor-specific T cells. J Immunother 2010; 33: 1–7.
Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med 2013; 368: 1509–1518.
Grupp SA, Maude SL, Shaw PA, Aplenc R, Barrett DM, Callahan C et al. Durable remissions in children with relapsed/refractory ALL treated with T cells engineered with a CD19-targeted chimeric antigen receptor (CTL019). Blood 2015; 126: Abstract 681.
Park JH, Riviere I, Wang X, Bernal Y, Purdon T, Halton E et al. Implications of minimal residual disease negative complete remission (MRD-CR) and allogeneic stem cell transplant on safety and clinical outcome of CD19-targeted 19-28z CAR modified T cells in adult patients with relapsed, refractory B-cell ALL. Blood 2015; 126: Abstract 682.
Lee DW, Stetler-Stevenson M, Yuan CM, Fry TJ, Shah NN, Delbrook C et al. Safety and response of incorporating CD19 chimeric antigen receptor T cell therapy in typical salvage regimens for children and young adults with acute lymphoblastic leukemia. Blood 2015; 126: Abstract 684.
Cruz CR, Micklethwaite KP, Savoldo B, Ramos CA, Lam S, Ku S et al. Infusion of donor-derived CD19-redirected virus-specific T cells for B-cell malignancies relapsed after allogeneic stem cell transplant: a phase 1 study. Blood 2013; 122: 2965–2973.
Turtle CJ, Hanafi LA, Berger C, Gooley TA, Cherian S, Hudecek M et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest 2016; 126: 2123–2138.
Gardner RA, Finney O, Smithers H, Leger K, Annesley CE, Summers C et al. Prolonged functional persistence of CD19CAR T cell products of defined CD4:CD8 composition and transgene expression determines durability of MRD-negative ALL remission. J Clin Oncol 2016; 34: Abstract 3048.
Teachey DT, Lacey SF, Shaw PA, Melenhorst JJ, Maude SL, Frey N et al. Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Cancer Discov 2016; 6: 664–679.
Fraietta JA, Beckwith KA, Patel PR, Ruella M, Zheng Z, Barrett DM et al. Ibrutinib enhances chimeric antigen receptor T-cell engraftment and efficacy in leukemia. Blood 2016; 127: 1117–1127.
Ruella M, Kenderian SS, Shestova O, Klichinsky M, Melenhorst JJ, Wasik MA et al. Kinase inhibitor ibrutinib to prevent cytokine-release syndrome after anti-CD19 chimeric antigen receptor T cells for B-cell neoplasms. Leukemia 2017; 31: 246–248.
Wang X, Wong CW, Urak R, Mardiros A, Budde LE, Chang WC et al. CMVpp65 vaccine enhances the antitumor efficacy of adoptively transferred CD19-redirected CMV-specific T cells. Clin Cancer Res 2015; 21: 2993–3002.
Terakura S, Yamamoto TN, Gardner RA, Turtle CJ, Jensen MC, Riddell SR . Generation of CD19-chimeric antigen receptor modified CD8+ T cells derived from virus-specific central memory T cells. Blood 2012; 119: 72–82.
Cooper LJ, Al-Kadhimi Z, Serrano LM, Pfeiffer T, Olivares S, Castro A et al. Enhanced antilymphoma efficacy of CD19-redirected influenza MP1-specific CTLs by cotransfer of T cells modified to present influenza MP1. Blood 2005; 105: 1622–1631.
Sun J, Huye LE, Lapteva N, Mamonkin M, Hiregange M, Ballard B et al. Early transduction produces highly functional chimeric antigen receptor-modified virus-specific T-cells with central memory markers: a production assistant for cell therapy (PACT) translational application. J Immunother Cancer 2015; 3: 5.
Budde LE, Berger C, Lin Y, Wang J, Lin X, Frayo SE et al. Combining a CD20 chimeric antigen receptor and an inducible caspase 9 suicide switch to improve the efficacy and safety of T cell adoptive immunotherapy for lymphoma. PLoS One 2013; 8: e82742.
Hoyos V, Savoldo B, Quintarelli C, Mahendravada A, Zhang M, Vera J et al. Engineering CD19-specific T lymphocytes with interleukin-15 and a suicide gene to enhance their anti-lymphoma/leukemia effects and safety. Leukemia 2010; 24: 1160–1170.
Wang X, Chang WC, Wong CW, Colcher D, Sherman M, Ostberg JR et al. A transgene-encoded cell surface polypeptide for selection, in vivo tracking, and ablation of engineered cells. Blood 2011; 118: 1255–1263.
Gardner R, Wu D, Cherian S, Fang M, Hanafi LA, Finney O et al. Acquisition of a CD19-negative myeloid phenotype allows immune escape of MLL-rearranged B-ALL from CD19 CAR-T-cell therapy. Blood 2016; 127: 2406–2410.
Sotillo E, Barrett DM, Black KL, Bagashev A, Oldridge D, Wu G et al. Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discov 2015; 5: 1282–1295.
Zah E, Lin MY, Silva-Benedict A, Jensen MC, Chen YY . T cells expressing CD19/CD20 bispecific chimeric antigen receptors prevent antigen escape by malignant B cells. Cancer Immunol Res 2016; 4: 498–508.
Ruella M, Barrett DM, Kenderian SS, Shestova O, Hofmann TJ, Perazzelli J et al. Dual CD19 and CD123 targeting prevents antigen-loss relapses after CD19-directed immunotherapies. J Clin Invest 2016; 126: 3814–3826.
Raponi S, De Propris MS, Intoppa S, Milani ML, Vitale A, Elia L et al. Flow cytometric study of potential target antigens (CD19, CD20, CD22, CD33) for antibody-based immunotherapy in acute lymphoblastic leukemia: analysis of 552 cases. Leuk Lymphoma 2011; 52: 1098–1107.
Kantarjian HM, DeAngelo DJ, Stelljes M, Martinelli G, Liedtke M, Stock W et al. Inotuzumab ozogamicin versus standard therapy for acute lymphoblastic leukemia. N Engl J Med 2016; 375: 740–753.
Maude SL, Barrett DM, Ambrose DE, Rheingold SR, Aplenc R, Teachey DT et al. Efficacy and safety of humanized chimeric antigen receptor (CAR)-modified T cells targeting CD19 in children with relapsed/refractory ALL. Blood 2015; 126: Abstract 683.
Rheingold SR, Chen LN, Maude SL, Aplenc R, Barker C, Barrett DM et al. Efficient trafficking of chimeric antigen receptor (CAR)-modified T cells to CSF and induction of durable CNS remissions in children with CNS/combined relapsed/refractory ALL. Blood 2015; 126: Abstract 3769.
Acknowledgements
The authors thank Erica S Chevalier-Larsen, PhD (Complete Healthcare Communications Inc, Chadds Ford, PA), whose work was funded by Amgen Inc, for assistance in preparing an outline and compiling the reference list; Janice Y Ahn, PhD (Amgen Inc) for editorial assistance; and Heather Hartley-Thorne (Sephirus Communications Inc), whose work was funded by Amgen Inc, for graphics assistance. Research support: Blinatumomab studies were funded by Amgen Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
IA has received consultancy fees from Helocyte Inc. RCB has received patents/royalties for blinatumomab from Amgen Inc and consultancy fees from Amgen Inc, Novartis, Pfizer, AstraZeneca, and GEMoaB GmbH. DN and GRF are currently employed by and hold stock in Amgen Inc; DN is an inventor on blinatumomab-related patents from Amgen Inc. PAB has been an employee of and holds stock in Amgen Inc. SJF reports clinical trial support from Amgen Inc and licensed patents for CAR development related to CD123 on AML and IL13ra2 on brain tumors to Mustang Therapeutics.
Rights and permissions
About this article
Cite this article
Aldoss, I., Bargou, R., Nagorsen, D. et al. Redirecting T cells to eradicate B-cell acute lymphoblastic leukemia: bispecific T-cell engagers and chimeric antigen receptors. Leukemia 31, 777–787 (2017). https://doi.org/10.1038/leu.2016.391
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2016.391
This article is cited by
-
CAR-T cell potency: from structural elements to vector backbone components
Biomarker Research (2022)
-
Direct control of CAR T cells through small molecule-regulated antibodies
Nature Communications (2021)
-
Dissecting the biology of allogeneic HSCT to enhance the GvT effect whilst minimizing GvHD
Nature Reviews Clinical Oncology (2020)
-
Treatment response, survival, safety, and predictive factors to chimeric antigen receptor T cell therapy in Chinese relapsed or refractory B cell acute lymphoblast leukemia patients
Cell Death & Disease (2020)
-
NG2 antigen is a therapeutic target for MLL-rearranged B-cell acute lymphoblastic leukemia
Leukemia (2019)