Abstract
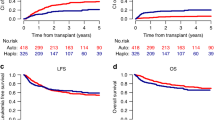
The optimal timing of allogeneic hematopoietic stem cell transplantation (HCT) in acute myeloid leukemia (AML) is controversial. We report on 1179 patients with a median age of 48 years who were randomized upfront. In the control arm, sibling HCT was scheduled in the first complete remission for intermediate-risk or high-risk AML and matched unrelated HCT in complex karyotype AML. In the experimental arm, matched unrelated HCT in first remission was offered also to patients with an FLT3-ITD (FMS-like tyrosine kinase 3–internal tandem duplication) allelic ratio >0.8, poor day +15 marrow blast clearance and adverse karyotypes. Further, allogeneic HCT was recommended in high-risk AML to be performed in aplasia after induction chemotherapy. In the intent-to-treat (ITT) analysis, superiority of the experimental transplant strategy could not be shown with respect to overall survival (OS) or event-free survival. As-treated analyses suggest a profound effect of allogeneic HCT on OS (HR 0.73; P=0.002) and event-free survival (HR 0.67; P<0.001). In high-risk patients, OS was significantly improved after allogeneic HCT in aplasia (HR 0.64; P=0.046) and after HCT in remission (HR 0.74; P=0.03). Although superiority of one study arm could not be demonstrated in the ITT analysis, secondary analyses suggest that early allogeneic HCT is a promising strategy for patients with high-risk AML.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Slovak ML, Kopecky KJ, Cassileth PA, Harrington DH, Theil KS, Mohamed A et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood 2000; 96: 4075–4083.
Suciu S, Mandelli F, de Witte T, Zittoun R, Gallo E, Labar B et al. Allogeneic compared with autologous stem cell transplantation in the treatment of patients younger than 46 years with acute myeloid leukemia (AML) in first complete remission (CR1): an intention-to-treat analysis of the EORTC/GIMEMAAML-10 trial. Blood 2003; 102: 1232–1240.
Burnett AK, Wheatley K, Goldstone AH, Stevens R, Hann I, Hills RK . Long-term results of the MRC AML10 trial. Clin Adv Hematol Oncol 2006; 4: 445–451.
Cornelissen JJ, van Putten WL, Verdonck LF, Theobald M, Jacky E, Daenen SM et al. Results of a HOVON/SAKK donor versus no-donor analysis of myeloablative HLA-identical sibling stem cell transplantation in first remission acute myeloid leukemia in young and middle-aged adults: benefits for whom? Blood 2007; 109: 3658–3666.
Koreth J, Schlenk R, Kopecky KJ, Honda S, Sierra J, Djulbegovic BJ et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. JAMA 2009; 301: 2349–2361.
Schetelig J, Bornhauser M, Schmid C, Hertenstein B, Schwerdtfeger R, Martin H et al. Matched unrelated or matched sibling donors result in comparable survival after allogeneic stem-cell transplantation in elderly patients with acute myeloid leukemia: a report from the cooperative German Transplant Study Group. J Clin Oncol 2008; 26: 5183–5191.
Gupta V, Tallman MS, He W, Logan BR, Copelan E, Gale RP et al. Comparable survival after HLA-well-matched unrelated or matched sibling donor transplantation for acute myeloid leukemia in first remission with unfavorable cytogenetics at diagnosis. Blood 2010; 116: 1839–1848.
Woolfrey A, Lee SJ, Gooley TA, Malkki M, Martin PJ, Pagel JM et al. HLA-allele matched unrelated donors compared to HLA-matched sibling donors: role of cell source and disease risk category. Biol Blood Marrow Transplant 2010; 16: 1382–1387.
Gratwohl A, Baldomero H, Schwendener A, Rocha V, Apperley J, Frauendorfer K et al. The EBMT activity survey 2007 with focus on allogeneic HSCT for AML and novel cellular therapies. Bone Marrow Transplant 2009; 43: 275–291.
Platzbecker U, Thiede C, Freiberg-Richter J, Rollig C, Helwig A, Schakel U et al. Early allogeneic blood stem cell transplantation after modified conditioning therapy during marrow aplasia: stable remission in high-risk acute myeloid leukemia. Bone Marrow Transplant 2001; 27: 543–546.
Platzbecker U, Thiede C, Fussel M, Geissler G, Illmer T, Mohr B et al. Reduced intensity conditioning allows for up-front allogeneic hematopoietic stem cell transplantation after cytoreductive induction therapy in newly-diagnosed high-risk acute myeloid leukemia. Leukemia 2006; 20: 707–714.
Schaich M, Parmentier S, Kramer M, Illmer T, Stolzel F, Rollig C et al. High-dose cytarabine consolidation with or without additional amsacrine and mitoxantrone in acute myeloid leukemia: results of the prospective randomized AML2003 trial. J Clin Oncol 2013; 31: 2094–2102.
Cassileth PA, Lynch E, Hines JD, Oken MM, Mazza JJ, Bennett JM et al. Varying intensity of postremission therapy in acute myeloid leukemia. Blood 1992; 79: 1924–1930.
Buchner T, Schlenk RF, Schaich M, Dohner K, Krahl R, Krauter J et al. Acute myeloid leukemia (AML): different treatment strategies versus a common standard arm–combined prospective analysis by the German AML Intergroup. J Clin Oncol 2012; 30: 3604–3610.
Thiede C, Steudel C, Mohr B, Schaich M, Schakel U, Platzbecker U et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood 2002; 99: 4326–4335.
Kern W, Haferlach T, Schoch C, Loffler H, Gassmann W, Heinecke A et al. Early blast clearance by remission induction therapy is a major independent prognostic factor for both achievement of complete remission and long-term outcome in acute myeloid leukemia: data from the German AML Cooperative Group (AMLCG) 1992 Trial. Blood 2003; 101: 64–70.
Pocock SJ, Simon R . Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics 1975; 31: 103–115.
Bornhauser M, Kienast J, Trenschel R, Burchert A, Hegenbart U, Stadler M et al. Reduced-intensity conditioning versus standard conditioning before allogeneic haemopoietic cell transplantation in patients with acute myeloid leukaemia in first complete remission: a prospective, open-label randomised phase 3 trial. Lancet Oncol 2012; 13: 1035–1044.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant 1995; 15: 825–828.
Atkinson K, Horowitz MM, Gale RP, Lee MB, Rimm AA, Bortin MM . Consensus among bone marrow transplanters for diagnosis, grading and treatment of chronic graft-versus-host disease. Committee of the International Bone Marrow Transplant Registry. Bone Marrow Transplant 1989; 4: 247–254.
Pocock SJ . Group sequential methods in the design and analysis of clinical trials. Biometrika 1977; 64: 191–199.
Simon R, Makuch RW . A non-parametric graphical representation of the relationship between survival and the occurrence of an event: application to responder versus non-responder bias. Stat Med 1984; 3: 35–44.
Lown RN, Shaw BE . Beating the odds: factors implicated in the speed and availability of unrelated haematopoietic cell donor provision. Bone Marrow Transplant 2013; 48: 210–219.
Bashey A, Zhang X, Sizemore CA, Manion K, Brown S, Holland HK et al. T-cell-replete HLA-haploidentical hematopoietic transplantation for hematologic malignancies using post-transplantation cyclophosphamide results in outcomes equivalent to those of contemporaneous HLA-matched related and unrelated donor transplantation. J Clin Oncol 2013; 31: 1310–1316.
Eapen M, Rocha V, Sanz G, Scaradavou A, Zhang MJ, Arcese W et al. Effect of graft source on unrelated donor haemopoietic stem-cell transplantation in adults with acute leukaemia: a retrospective analysis. Lancet Oncol 2010; 11: 653–660.
Eapen M, O'Donnell P, Brunstein CG, Wu J, Barowski K, Mendazibal A et al. Mismatched related and unrelated donors for allogeneic hematopoietic cell transplantation for adults with hematologic malignancies. Biol Blood Marrow Transplant 2014; 20: 1485–1492.
Mantel N, Byar DP . Evaluation of response-time data involving transient states: an illustration using heart-transplant data. Biometrics 1974; 69: 81–86.
Burnett AK . Treatment of acute myeloid leukemia: are we making progress? Hematology Am Soc Hematol Educ Program 2012; 2012: 1–6.
Craddock C, Labopin M, Pillai S, Finke J, Bunjes D, Greinix H et al. Factors predicting outcome after unrelated donor stem cell transplantation in primary refractory acute myeloid leukaemia. Leukemia 2011; 25: 808–813.
Duval M, Klein JP, He W, Cahn JY, Cairo M, Camitta BM et al. Hematopoietic stem-cell transplantation for acute leukemia in relapse or primary induction failure. J Clin Oncol 2010; 28: 3730–3738.
Schmid C, Schleuning M, Ledderose G, Tischer J, Kolb HJ . Sequential regimen of chemotherapy, reduced-intensity conditioning for allogeneic stem-cell transplantation, and prophylactic donor lymphocyte transfusion in high-risk acute myeloid leukemia and myelodysplastic syndrome. J Clin Oncol 2005; 23: 5675–5687.
Schmid C, Schleuning M, Hentrich M, Markl GE, Gerbitz A, Tischer J et al. High antileukemic efficacy of an intermediate intensity conditioning regimen for allogeneic stem cell transplantation in patients with high-risk acute myeloid leukemia in first complete remission. Bone Marrow Transplant 2008; 41: 721–727.
Warlick ED, Paulson K, Brazauskas R, Zhong X, Miller AM, Camitta BM et al. Effect of postremission therapy before reduced-intensity conditioning allogeneic transplantation for acute myeloid leukemia in first complete remission. Biol Blood Marrow Transplant 2014; 20: 202–208.
Acknowledgements
We thank the support of the DKMS, which allowed for immediate HLA-typing and provisional unrelated donor search. Further, we thank the data managers of the central study office of the Study Alliance Leukemia, especially Annett Engmann and Katrin Peschel for their extensive work and their excellent contributions to this project.
Author Contributions
Financial and administrative support: Gerhard Ehninger. Conception and design: Gerhard Ehninger, Markus Schaich, and Martin Bornhäuser. Collection and assembly of data: Kerstin Schäfer-Eckart, Matthias Hänel, Walter E Aulitzky, Hermann Einsele, Norbert Schmitz, Wolf Rösler, Matthias Stelljes, Claudia D Baldus, Anthony D Ho, Andreas Neubauer, Hubert Serve, Stefani Parmentier, Christoph Röllig, Uwe Platzbecker, Jiri Mayer, Wolfgang E Berdel, Christian Thiede, and Markus Schaich. Central diagnostics: Christian Thiede, Brigitte Mohr, Uta Oelschlägel, and Stefani Parmentier. Statistical analysis: Michael Kramer and Johannes Schetelig. Interpretation: Gerhard Ehninger, Martin Bornhäuser, Markus Schaich and Johannes Schetelig. Manuscript writing: Johannes Schetelig and Martin Bornhäuser. Final approval of manuscript: All coauthors reviewed and approved the final manuscript.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
Schetelig, J., Schaich, M., Schäfer-Eckart, K. et al. Hematopoietic cell transplantation in patients with intermediate and high-risk AML: results from the randomized Study Alliance Leukemia (SAL) AML 2003 trial. Leukemia 29, 1060–1068 (2015). https://doi.org/10.1038/leu.2014.335
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2014.335
This article is cited by
-
UBTF tandem duplications are rare but recurrent alterations in adult AML and associated with younger age, myelodysplasia, and inferior outcome
Blood Cancer Journal (2023)
-
Indications for haematopoietic cell transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2022
Bone Marrow Transplantation (2022)
-
Morphologic leukemia-free state in acute myeloid leukemia is sufficient for successful allogeneic hematopoietic stem cell transplant
Blood Cancer Journal (2021)
-
miR-10a as a therapeutic target and predictive biomarker for MDM2 inhibition in acute myeloid leukemia
Leukemia (2021)
-
Comparison analysis between haplo identical stem cell transplantation and matched sibling donor stem cell transplantation for high-risk acute myeloid leukemia in first complete remission
Science China Life Sciences (2019)