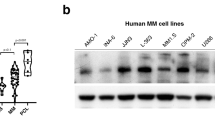
Abstract
DNA double-strand breaks (DSBs) are deleterious lesions that can lead to chromosomal anomalies, genomic instability and cancer. The histone protein H2AX has an important role in the DNA damage response (DDR) and the presence of phospho-H2AX (γH2AX) nuclear foci is the hallmark of DSBs. We hypothesize that ongoing DNA damage provides a mechanism by which chromosomal abnormalities and intratumor heterogeneity are acquired in malignant plasma cells (PCs) in patients with multiple myeloma (MM). Therefore, we assessed PCs from patients with the premalignant condition, monoclonal gammopathy of undetermined significance (MGUS) and MM, as well as human MM cell lines (HMCLs) for evidence of DSBs. γH2AX foci were detected in 2/5 MGUS samples, 37/40 MM samples and 6/6 HMCLs. Notably, the DSB response protein 53BP1 colocalized with γH2AX in both MM patient samples and HMCLs. Treatment with wortmannin decreased phosphorylation of H2AX and suggests phosphoinositide (PI) 3-kinases and/or PI3-kinase-like family members underlie the presence of γH2AX foci in MM cells. Taken together, these data imply that ongoing DNA damage intensifies across the disease spectrum of MGUS to MM and may provide a mechanism whereby clonal evolution occurs in the monoclonal gammopathies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Raab MS, Podar K, Breitkreutz I, Richardson PG, Anderson KC . Multiple myeloma. Lancet 2009; 374: 324–339.
Landgren O, Kyle RA, Pfeiffer RM, Katzmann JA, Caporaso NE, Hayes RB et al. Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: a prospective study. Blood 2009; 113: 5412–5417.
Weiss BM, Abadie J, Verma P, Howard RS, Kuehl WM . A monoclonal gammopathy precedes multiple myeloma in most patients. Blood 2009; 113: 5418–5422.
Kyle RA, Rajkumar SV . Monoclonal gammopathies of undetermined significance. Rev Clin Exp Hematol 2002; 6: 225–252.
Bergsagel PL, Kuehl WM . Molecular pathogenesis and a consequent classification of multiple myeloma. J Clin Oncol 2005; 23: 6333–6338.
Pasqualucci L, Neumeister P, Goossens T, Nanjangud G, Chaganti RS, Kuppers R et al. Hypermutation of multiple proto-oncogenes in B-cell diffuse large-cell lymphomas. Nature 2001; 412: 341–346.
Gabrea A, Leif Bergsagel P, Michael Kuehl W . Distinguishing primary and secondary translocations in multiple myeloma. DNA Repair (Amst) 2006; 5: 1225–1233.
Gorgoulis VG, Vassiliou LV, Karakaidos P, Zacharatos P, Kotsinas A, Liloglou T et al. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature 2005; 434: 907–913.
McKinnon PJ, Caldecott KW . DNA strand break repair and human genetic disease. Annu Rev Genomics Hum Genet 2007; 8: 37–55.
Bonner WM, Redon CE, Dickey JS, Nakamara AJ, Sedelnikova OA, Solier S et al. GammaH2AX and cancer. Nat Rev Cancer 2008; 8: 957–967.
Kinner A, Wu W, Staudt C, Iliakis G . Gamma-H2AX in recognition and signaling of DNA double-strand breaks in the context of chromatin. Nucleic Acids Res 2008; 36: 5678–5694.
Rogakou EP, Boon C, Redon C, Bonner WM . Megabase chromatin domains involved in DNA double-strand breaks in vivo. J Cell Biol 1999; 146: 905–916.
Muslimovic A, Ismail IH, Gao Y, Hammarsten O . An optimized method for measurement of gamma-H2AX in blood mononuclear and cultured cells. Nat Protoc 2008; 3: 1187–1193.
Kruhlak MJ, Celeste A, Dellaire G, Fernandez-Capetillo O, Muller WG, McNally JG et al. Changes in chromatin structure and mobility in living cells at sites of DNA double-strand breaks. J Cell Biol 2006; 172: 823–834.
Paull TT, Rogakou EP, Yamazaki V, Kirchgessner CU, Gellert M, Bonner WM . A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr Biol 2000; 10: 886–895.
Pilch DR, Sedelnikova OA, Redon C, Celeste A, Nussenzweig A, Bonner WM . Characteristics of gamma-H2AX foci at DNA double-strand breaks sites. Biochem Cell Biol 2003; 81: 123–129.
Bartkova J, Horejsi Z, Koed K, Kramer A, Tort F, Zieger K et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature 2005; 434: 864–870.
Bartkova J, Hamerlik P, Stockhausen MT, Ehrmann J, Hlobilkova A, Laursen H et al. Replication stress and oxidative damage contribute to aberrant constitutive activation of DNA damage signalling in human gliomas. Oncogene 2010; 29: 5095–5102.
Warters RL, Adamson PJ, Pond CD, Leachman SA . Melanoma cells express elevated levels of phosphorylated histone H2AX foci. J Invest Dermatol 2005; 124: 807–817.
Dai Y, Chen S, Pei XY, Almenara JA, Kramer LB, Venditti CA et al. Interruption of the Ras/MEK/ERK signaling cascade enhances Chk1 inhibitor-induced DNA damage in vitro and in vivo in human multiple myeloma cells. Blood 2008; 112: 2439–2449.
Kiziltepe T, Hideshima T, Ishitsuka K, Ocio EM, Raje N, Catley L et al. JS-K, a GST-activated nitric oxide generator, induces DNA double-strand breaks, activates DNA damage response pathways, and induces apoptosis in vitro and in vivo in human multiple myeloma cells. Blood 2007; 110: 709–718.
Ocio EM, Maiso P, Chen X, Garayoa M, Alverez-Fernandez S, San Segundo L et al. Zalypsis: a novel marine-derived compound with potent antimyeloma activity that reveals high sensitivity of malignant plasma cells to DNA double-strand breaks. Blood 2009; 113: 3781–3791.
Yang C, Betti C, Singh S, Toor A, Vaughan A . Impaired NHEJ function in multiple myeloma. Mutat Res 2009; 660: 66–73.
Kyle RA, Rajkumar SV . Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia 2009; 23: 3–9.
Westendorf JJ, Ahmann GJ, Greipp PR, Witzig TE, Lust JA, Jelinek DF . Establishment and characterization of three myeloma cell lines that demonstrate variable cytokine responses and abilities to produce autocrine interleukin-6. Leukemia 1996; 10: 866–876.
Jelinek DF, Ahmann GJ, Greipp PR, Jalal SM, Westendorf JJ, Katzmann JA et al. Coexistence of aneuploid subclones within a myeloma cell line that exhibits clonal immunoglobulin gene rearrangement: clinical implications. Cancer Res 1993; 53: 5320–5327.
Arendt BK, Ramirez-Alvarado M, Sikkink LA, Keats JJ, Ahmann GJ, Dispenzieri A et al. Biologic and genetic characterization of the novel amyloidogenic lambda light chain-secreting human cell lines, ALMC-1 and ALMC-2. Blood 2008; 112: 1931–1941.
Greipp PR, Kumar S . Plasma cell labeling index. Methods Mol Med 2005; 113: 25–35.
Walters DK, French JD, Arendt BK, Jelinek DF . Atypical expression of ErbB3 in myeloma cells: cross-talk between ErbB3 and the interferon-alpha signaling complex. Oncogene 2003; 22: 3598–3607.
Arora T, Jelinek DF . Differential myeloma cell responsiveness to interferon-alpha correlates with differential induction of p19(INK4d) and cyclin D2 expression. J Biol Chem 1998; 273: 11799–11805.
Resnick MA, Martin P . The repair of double-strand breaks in the nuclear DNA of Saccharomyces cerevisiae and its genetic control. Mol Gen Genet 1976; 143: 119–129.
MacPhail SH, Banath JP, Yu Y, Chu E, Olive PL . Cell cycle-dependent expression of phosphorylated histone H2AX: reduced expression in unirradiated but not X-irradiated G1-phase cells. Radiat Res 2003; 159: 759–767.
Celeste A, Petersen S, Romanienko PJ, Fernandez-Capetillo O, Chen HT, Sedelnikova OA et al. Genomic instability in mice lacking histone H2AX. Science 2002; 296: 922–927.
DiTullio Jr RA, Mochan TA, Venere M, Bartkova J, Sehested M, Bartek J et al. 53BP1 functions in an ATM-dependent checkpoint pathway that is constitutively activated in human cancer. Nat Cell Biol 2002; 4: 998–1002.
Shiotani B, Zou L . Single-stranded DNA orchestrates an ATM-to-ATR switch at DNA breaks. Mol Cell 2009; 33: 547–558.
Sarkaria JN, Tibbetts RS, Busby EC, Kennedy AP, Hill DE, Abraham RT . Inhibition of phosphoinositide 3-kinase related kinases by the radiosensitizing agent wortmannin. Cancer Res 1998; 58: 4375–4382.
Marusyk A, DeGregori J . Replicational stress selects for p53 mutation. Cell Cycle 2007; 6: 2148–2151.
Collins SJ . The HL-60 promyelocytic leukemia cell line: proliferation, differentiation, and cellular oncogene expression. Blood 1987; 70: 1233–1244.
Yu T, MacPhail SH, Banath JP, Klokov D, Olive PL . Endogenous expression of phosphorylated histone H2AX in tumors in relation to DNA double-strand breaks and genomic instability. DNA Repair (Amst) 2006; 5: 935–946.
Osborn AJ, Elledge SJ, Zou L . Checking on the fork: the DNA-replication stress-response pathway. Trends Cell Biol 2002; 12: 509–516.
Andreassen PR, D’Andrea AD, Taniguchi T . ATR couples FANCD2 monoubiquitination to the DNA-damage response. Genes Dev 2004; 18: 1958–1963.
Ward IM, Chen J . Histone H2AX is phosphorylated in an ATR-dependent manner in response to replicational stress. J Biol Chem 2001; 276: 47759–47762.
Jirmanova L, Bulavin DV, Fornace Jr AJ . Inhibition of the ATR/Chk1 pathway induces a p38-dependent S-phase delay in mouse embryonic stem cells. Cell Cycle 2005; 4: 1428–1434.
Chanoux RA, Yin B, Urtishak KA, Asare A, Bassing CH, Brown EJ . ATR and H2AX cooperate in maintaining genome stability under replication stress. J Biol Chem 2009; 284: 5994–6003.
Efeyan A, Serrano M . p53: guardian of the genome and policeman of the oncogenes. Cell Cycle 2007; 6: 1006–1010.
Neri A, Baldini L, Trecca D, Cro L, Polli E, Maiolo AT . p53 gene mutations in multiple myeloma are associated with advanced forms of malignancy. Blood 1993; 81: 128–135.
Acknowledgements
This work was supported by a grant from the National Institutes of Health (CA062242).
Author contributions
DKW designed and performed research, analyzed data and wrote the manuscript. RCT, BKA and KJH performed research and analyzed data. XW designed research and interpreted data. PMH and AD designed research and enrolled patients. DFJ designed research, wrote and approved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Walters, D., Wu, X., Tschumper, R. et al. Evidence for ongoing DNA damage in multiple myeloma cells as revealed by constitutive phosphorylation of H2AX. Leukemia 25, 1344–1353 (2011). https://doi.org/10.1038/leu.2011.94
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2011.94
Keywords
This article is cited by
-
Preclinical validation and phase I trial of 4-hydroxysalicylanilide, targeting ribonucleotide reductase mediated dNTP synthesis in multiple myeloma
Journal of Biomedical Science (2022)
-
A drug repurposing strategy for overcoming human multiple myeloma resistance to standard-of-care treatment
Cell Death & Disease (2022)
-
TRIM44 mediated p62 deubiquitination enhances DNA damage repair by increasing nuclear FLNA and 53BP1 expression
Oncogene (2021)
-
A novel M phase blocker, DCZ3301 enhances the sensitivity of bortezomib in resistant multiple myeloma through DNA damage and mitotic catastrophe
Journal of Experimental & Clinical Cancer Research (2020)
-
A novel BCMA PBD-ADC with ATM/ATR/WEE1 inhibitors or bortezomib induce synergistic lethality in multiple myeloma
Leukemia (2020)