Abstract
Objective:
To determine whether prenatal treatment with a single course of glucocorticoids (GCs) affects size at birth among full-term infants independent of fetal size before GC administration or exposure to preterm labor (PTL).
Study Design:
In all, 105 full-term infants were recruited into three study groups (30 GC treated; 60 controls matched for gestational age (GA) at birth and sex; and 15 PTL controls without GC exposure). Size of the infants was estimated before treatment using two-dimensional (2D) ultrasound and by direct measurement at birth.
Results:
Length, weight and head circumference at birth were smaller among GC-treated infants compared with matched controls (P's<0.01), although fetal size did not differ before treatment (P's>0.2). Exposure to PTL did not account for this effect.
Conclusions:
Prenatal treatment with a single course of GCs was associated with a reduction in size at birth among infants born at term gestation. This effect cannot be explained by differences in fetal size before treatment or exposure to PTL.
Similar content being viewed by others
Introduction
Following the National Institutes of Health (NIH) consensus statement in 1995, endorsing the use of prenatal glucocorticoid (GC) administration to accelerate fetal lung maturation,1 there was a steady increase in the use of this intervention for mothers at risk of preterm delivery. Evidence has shown that treatment with GCs decreases respiratory distress syndrome and increases survival among infants born preterm.2 Given these clear benefits for preterm infants, it is widely acknowledged that the benefits outweigh the associated risks. As a result prenatal GC therapy is now considered to be the standard of care for women who are at risk of preterm delivery. In fact, a common quality of care indicator used in birthing hospitals in North America and Europe is the proportion of women admitted for preterm labor (PTL) who receive prenatal GC treatment. As the clinical diagnosis of PTL is imprecise and because preterm delivery may be prevented with clinical interventions, over one-third of the women diagnosed as being in PTL deliver at term.3 Further, there is evidence that the exposure of full-term infants to prenatal GCs is on the rise.4
Findings from animal models have consistently demonstrated that a primary negative consequence of prenatal exposure to excess GCs is reduced size at birth.5, 6, 7, 8, 9 Further, such exposure appears to exert a programming influence on development with persisting consequences, including increased blood pressure and glucose intolerance in adulthood.10, 11, 12 Studies evaluating the effect of prenatal GC therapy on somatic growth among humans have focused on preterm infants and yielded conflicting results. Although some studies show reduced size at birth,13, 14, 15, 16 others show no effect of treatment.17 Consideration of the consequences of prenatal GC treatment on infants born at term gestation is important for the following reasons. First, existing studies evaluating the effects of prenatal GCs for size at birth are complicated by the fact that most of the infants studied were born before 37 weeks of gestation and were, therefore, already at risk for delayed growth and development. For example, data indicate that fetal growth is suppressed as early as the first trimester in pregnancies that subsequently deliver preterm.18 All of the studies showing no effect of prenatal GC therapy on fetal growth have included preterm infants. Second, for many infants born preterm there is only a short time interval between GC treatment and delivery that limits the ability to observe effects on size at birth.19 For example, if a preterm infant is born shortly after GC treatment, even if growth is affected, birth size would not be reduced. One published study reported that prenatal GC therapy administered to infants subsequently born at term, was associated with reduced size at birth.20 However, this study did not examine whether there were differences in fetal size before GC treatment or if exposure to PTL affected growth.
The goal of this study is to determine whether treatment with a single course of prenatal GCs is associated with size at birth among infants born at term gestation. We first compared full-term GC-treated infants to a comparison group of infants born at term gestation and matched for sex and gestational age (GA) at birth. Analyses were performed to assess the influence of fetal size before the administration of GCs and the effects of exposure to PTL.
Methods
Participants
The Institutional Review Board for protection of human subjects at the University of California, Irvine, approved the study protocol. Mother–infant pairs were recruited into a longitudinal study designed to examine the consequences of prenatal GC treatment on infant development. Written and informed consent was obtained before study enrollment. Inclusion criteria for these analyses were as follows: birth at term (>37 weeks' GA), singleton status and admission to the normal newborn nursery. Exclusion criteria were chromosomal or other congenital anomalies (e.g., trisomy 21), congenital infections and major neonatal illness (e.g., sepsis), as well as maternal disorders during pregnancy requiring corticosteroid treatment or thyroid medication, and an evidence of smoking during the prenatal period (based on maternal report) or an evidence of maternal substance use during pregnancy (e.g., alcohol). Eighty-five percent of the women approached agreed to participate in this study.
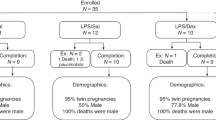
The study sample comprized 105 full-term infants born at the University of California, Irvine Medical Center, and were recruited into three groups. The GC group included 30 full-term infants (20 female) whose mothers received prenatal treatment with a single course of the GC, betamethasone (12 mg per dose for 2 doses 24 h apart). In this cohort the first dose of betamethasone was given between 25 and 34 weeks' GA (mean GA at administration=29.5 (2.9) weeks). The primary indication for prenatal GC administration was PTL (87%). PTL was defined based on the diagnosis by the attending obstetrician and when indicated in the chart. PTL was diagnosed by the attending obstetrician based on the following factors: cervical change over time, bloody show, cervical effacement and/or dilation and rupture of membranes. Other associated factors included placenta previa and prolonged premature rupture of membranes. The first comparison group (matched controls) consisted of 60 normal full-term infants without prenatal GC treatment or PTL (40 female) matched by GA at birth and sex to the prenatal GC-treated group. The second comparison group (PTL) comprized 15 full-term infants (10 female) whose mothers experienced documented PTL, as diagnosed by the attending obstetrician and delivered at term, but did not receive GCs. The GC group was recruited consecutively. Infants in the comparison groups were infants born concurrently with the target group who met the inclusion criteria for the relevant comparison group. To create a more stable characterization of size at birth among unexposed infants, two no-treatment controls were matched with each infant in the target group.
Procedures
The primary outcome variables, neonatal length, weight and head circumference at birth, were obtained from birth records. All infants were measured shortly after birth as part of their initial evaluation and standardized physical examination. The newborn infant was weighed, without any clothing, on an electronic scale, which was calibrated before each use. The infant was then immediately transferred to a radiant heat warmer, where the head circumference and length in centimeters was obtained using a disposable tape measure. All measurements were then noted on the infant's admission chart. These data were abstracted from the infant's medical records for the purpose of this investigation. Birth weight percentiles were determined based on data normalized for GA at birth and sex.21 Maternal medical records were reviewed to abstract information pertaining to birth, pregnancy characteristics and demographic data. In a subset of participants (75%), prenatal records were available for the assessment of fetal size (biparietal diameter, head circumference, femur length, abdominal circumference and estimated fetal weight) as determined by the routine two-dimensional (2D) ultrasound conducted between 16 and 22 weeks' GA.22
Statistical analyses
Preliminary analyses were performed to determine if groups differed on demographic or medical characteristics. Significant group differences were included as covariates in subsequent analyses. Analysis of covariance (ANCOVA) was used to address the primary study question of whether size at birth differed between the GC group and the matched controls. Linear regression was used to determine effects of timing of exposure to prenatal GCs. Additional analysis of covariances were carried out to evaluate fetal size before the administration of GCs and to compare the PTL group to the GC group and the matched controls.
Results
Demographics
As shown in Table 1, the prenatal GC treatment and matched control groups did not significantly differ in GA at birth, Apgar scores, mode of delivery, maternal age, parity, type of medical insurance as a proxy for socioeconomic status (SES) and GA at first prenatal visit. The difference in the number of Hispanic infants in the three groups approached a statistical significance (P=0.07). Race/ethnicity was entered as a covariate in all subsequent analyses.
Is prenatal treatment with a single course of GCs associated with size at birth among infants born at term gestation?
As shown in Table 2, prenatal GC-treated infants were significantly smaller at birth (both raw data and Z-scores) than controls matched for GA and sex in length (P<0.001), weight (P<0.001), birth weight percentile (P<0.001) and head circumference (P<0.01). Prenatal GC treatment accounted for 20% of the variance in length, 16% in weight, 14% in birth weight percentile and 12% in head circumference. Prenatal GC-treated infants were not significantly more likely to have birth weights below the 10th percentile (P=0.26).
Does timing of prenatal GC treatment moderate the association between GC exposure and size at birth?
Gestational age at GC administration was significantly associated with infant size at birth. Fetal exposure to prenatal GCs earlier in gestation was associated with a significantly greater decrease in body length (P<0.05) and a non-significant trend for a greater decrease in birth weight (P=0.10). No association was found between timing of administration and head circumference (P=0.36).
Possible alternative hypotheses
These data suggest that prenatal treatment with a single course of GCs is associated with decreased fetal growth. We performed two additional sets of analyses to rule out alternative explanations for these findings.
Hypothesis 1: fetal size differed before exposure to prenatal GCs
To evaluate pre-existing differences in fetal size among the groups, we examined the two-dimensional ultrasound data collected between 16 and 22 weeks' GA, as part of mother's routine prenatal care. The groups with and without ultrasound data did not significantly differ in any of the birth outcome measures or GA at birth, mode of delivery, sex, maternal age at delivery, race/ethnicity, parity and type of medical insurance or GA estimate at their first prenatal visit (all P's>0.2).
Fetal GA at the time of fetal ultrasound did not differ between the study groups (P=0.34). The prenatal GC group did not differ from the matched control group on estimated fetal weight or any other growth parameter (all P's>0.3; Table 3). These data demonstrate that fetal size was not different among the groups before the administration of GCs to the treatment group.
Analyses evaluating the effect of prenatal GC treatment on birth outcome were repeated with the subgroup of study participants (n=79) for whom prenatal ultrasound data was available. The pattern of results was identical to the findings in the complete sample. Specifically, at birth, length (47.5 vs 49.5 cm), weight (2914.7 vs 3375.6 g.), head circumference (33.2 vs 34.4 cm) and birth weight percentile (31.2 vs 54.6%) were smaller in the GC group when compared to the matched control group (P's<0.01), despite the absence of differences before prenatal GC exposure.
Hypothesis 2: group differences are due to exposure to PTL
To examine whether exposure to PTL accounted for these group differences, we recruited an additional 15 infants whose mothers had documented PTL and delivered at term, but did not receive GC treatment. Demographic information was not significantly different between the groups (Table 1). Furthermore, as shown in Table 2, the PTL group did not differ from the matched control group, born at term without exposure to prenatal GCs or PTL, on any of the birth outcome measures (all P's>0.3). It should be noted that the prenatal GC group was significantly smaller in terms of birth weight (P<0.01), length (P<0.01) and birth weight percentile (P<0.01), but not head circumference (P=0.5) as compared with the PTL group. Examination of the prenatal ultrasound data revealed that the PTL group did not differ in GA at the time of ultrasound, estimated fetal weight or any other fetal growth parameter (P>0.3) compared with the GC group or the matched control group (see Table 3).
Discussion
In 1995 the National Institutes of Health issued a consensus statement recommending that prenatal GC treatment be given to all women who are at a risk of preterm delivery between 24 and 34 weeks of gestation.1 Consequently the increased use of prenatal GC therapy and other interventions for PTL have led to an increasing number of treated infants being born at term gestation. This study is the first to assess the association between prenatal GC treatment and size at birth among infants born at term gestation after controlling for pretreatment fetal size and exposure to PTL. Our data demonstrating that prenatal GC treatment is associated with smaller size at birth, adds to concerns that have been raised regarding the developmental consequences of such exposure; concerns that are particularly relevant for exposed infants born at term who do not receive commiserate medical benefits from GC therapy. These data also suggest that there may be a period of vulnerability at the time of late second and early third trimesters during which fetal growth is more sensitive to the effects of prenatal GC treatment.
The suppressive effects of prenatal GC therapy on somatic growth in this population are consistent with models from a variety of species. Carefully conducted models with the sheep and the non-human primate provide clear evidence that size at birth is reduced as a consequence of prenatal exposure to multiple doses of GCs.5, 6, 9 Although some studies observe decreases in neonatal size at birth after only one dose8, 23 others do not.12 In contrast to animal models, studies with humans have focused on preterm infants and have yielded mixed results. Studies evaluating both single13, 14 and multiple15, 16, 24 courses have found retarding effects on human growth, although not all studies have found such an association.25, 26 The limitation of focusing on preterm infants is that it is not possible to determine if the effects on growth are a result of prenatal GC treatment or from consequences of intrauterine complications related to preterm delivery. Specifically, data indicate that fetal growth is already attenuated among infants who subsequently deliver preterm, thereby potentially masking effects of GC treatment.18 Furthermore, in many cases of PTL there is only a short time interval between GC administration and delivery, which may not allow sufficient time for the suppressive effects on growth to be observed at birth.19 Our findings of decreased body length, weight and head circumference among term infants exposed to prenatal GC treatment are consistent with the only previously published data, to evaluate the consequence of prenatal GC therapy on term infants.20 Our study has extended these findings in important ways by ruling out the possibility that these effects are due to pre-existing differences in fetal size or exposure to PTL.
Several limitations of this work should be acknowledged. First, participants were not randomly assigned to treatment and control groups. Second, assessments of size at birth were determined based on clinical assessments. Data from infants in the three groups were, however, collected contemporaneously and by trained neonatal personnel. It is expected that any measurement error is distributed evenly across groups. Third, fetal ultrasound data were collected only on a subset of infants. It should be noted that the same pattern of results regarding associations between GC treatment and size at birth was still observed when only those infants with prenatal ultrasound data were included.
There are several unique strengths that validate these results. In addition to the inclusion of only full-term infants, this is the first study to evaluate fetal size before prenatal GC administration. Previous work has shown that fetal growth is delayed as early as the first trimester among pregnancies that subsequently deliver preterm.18 Not only were all of the infants in this study born at term gestation, but using ultrasound measurements taken before prenatal GC administration, we were able to rule out the possibility that prenatal GC therapy was simply a marker of pre-existing differences between the study groups.
A second unique aspect of this study is the evidence that exposure to PTL, among term infants not receiving GC treatment, did not affect size at birth. This suggests that PTL by itself was not significantly associated with the attenuation of fetal growth. In addition, the GC group was smaller in terms of body length and weight at birth compared with controls, with and without PTL, indicating that exposure to prenatal GCs explains the effects on size at birth independent of effects of PTL. Results for head circumference are less clear as the PTL group did not differ from either the term controls or the GC group. The smaller head circumference among term GC-treated infants when compared with the matched controls may result from the cumulative effect of exposure to GCs and PTL. Adverse exposures that affect fetal growth during the third trimester, a period of cellular hypertrophy, typically result in the type of asymmetric growth restriction seen among the GC-treated infants, in which somatic growth is suppressed to a greater extent than cranial growth.27 Thus, the timing of GC exposure may result in a larger attenuation of somatic growth and the detection of effects that are independent of PTL as shown in this study. As there was no random assignment to study group, the possibility remains that infants exposed to PTL, but not GC treatment experienced a different prenatal course as compared with infants exposed to PTL, who received GCs. Nonetheless, these data provide further evidence that the effect of GC treatment on fetal growth cannot be explained by PTL.
Glucocorticoids appear to exert a programming influence on the developing organism.28 During pregnancy GCs are essential for the regulation of intrauterine homeostasis, and differentiation and maturation of vital organ systems including the lungs, the liver and the central nervous system.29, 30, 31, 32 Synthetic steroids readily pass through the placenta.33, 34 They are not oxidized by 11-beta-hydroxysteroid dehydrogenase type 2, as is maternal cortisol, and have a greater affinity for naturally occurring receptors than cortisol.35 Thus, it is likely that the administration of a large dose of GCs during pregnancy could have consequences on multiple organ systems besides the lung. Animal models have, in fact, shown that excess GCs cause both reduced neonatal size at birth and impaired health in adulthood.10, 11, 12 The mechanisms underlying GC effects of fetal growth in humans are unknown. Animal models have shown that the fetal insulin-like growth factor axis is altered by exposure to excess GCs and that alterations to this system are related to suppression of fetal growth.36
Although the current findings provide evidence for attenuation of fetal growth associated with GC treatment it cannot be determined whether these are transient effects or if there are long-term consequences for health and development. The long-term consequences of GC treatment for human development remain under investigation. A recent 30-year follow-up of a randomized controlled trial of GC therapy did not find effects on adult body size, blood pressure, blood lipids or plasma cortisol.25 Increased insulin resistance was, however, noted among individuals exposed to prenatal GC therapy, thus, providing some evidence of a programming effect of prenatal GCs.
The beneficial effects of GC treatment for preterm infants are clearly established. Equivalent benefits are not conferred on those infants born at term. Our data demonstrate measureable effects on fetal growth parameters among term infants with prenatal GC exposure, and suggest a potential negative consequence for a group that does not receive commiserate medical benefit from treatment. As the clinical diagnosis of PTL is difficult a significant number of women receive GC treatment (as well as other interventions) and go on to deliver beyond 37-weeks gestation. The potential harm to term infants must be evaluated in the context of the clear benefit to preterm infants. Thus, these data do not suggest that GC treatment should not be administered, but rather emphasize the importance of improving the accuracy of diagnosis of PTL to reduce the exposure of infants subsequently born at term to prenatal GC treatment. The evidence that a single course of GCs is associated with a reduction in size at birth further substantiates the case against multiple doses. If a single course of GC treatment has negative implications for fetal growth, it is plausible that multiple doses will incur even greater consequences.
Conflict of interest
The authors declare no conflict of interest.
References
NIH Consensus Development Conference. Effects of corticosteroids for fetal maturation and perinatal outcomes. Am J Obstet Gynecol 1995; 173: 253–344.
Crowley P . Antenatal corticosteroid therapy: a meta-analysis of the randomized trials, 1972–1994. Am J Obstet Gynecol 1995; 173: 322–355.
Steer P, Flint C . ABC of labour care: preterm labour and premature rupture of membranes. Br Med J 1999; 318: 1059–1062.
Polyakov A, Cohen S, Baum M, Trickey D, Jolley D, Wallace EM . Patterns of antenatal corticosteroid prescribing 1998–2004. Aust N Z J Obstet Gynaecol 2007; 47 (1): 42–45.
Moss TJ, Nitsos I, Harding R, Newnham JP . Differential effects of maternal and fetal betamethasone injections in late-gestation fetal sheep. J Soc Gynecol Investig 2003; 10 (8): 474–479.
Johnson J, Mitzner W, London W, Palmer A, Scott R . Betamethasone and the rhesus fetus: multisystemic effects. Am J Obstet Gynecol 1979; 133 (6): 677–684.
Kutzler MA, Ruane EK, Coksaygan T, Vincent SE, Nathanielsz PW . Effects of three courses of maternally administered dexamethasone at 0.7, 0.75, and 0.8 of gestation on prenatal and postnatal growth in sheep. Pediatrics 2004; 113 (2): 313–319.
Jobe A, Wada N, Berry L, Ikegami M, Ervin M . Single and repetitive maternal glucocorticoid exposure reduce fetal growth in sheep. Am J Obstet Gynecol 1998; 178: 880–885.
Johnson JW, Mitzner W, London WT, Sly DL, Lee PA, Khouzami VA et al. Long-term effects of betamethasone on fetal development. Am J Obstet Gynecol 1981; 141: 1053–1064.
Levitt NS, Lindsay RS, Holmes MC, Seckl JR . Dexamethasone in the last week of pregnancy attenuates hippocampal glucocorticoid receptor gene expression and elevates blood pressure in the adult offspring in the rat. Neuroendocrinology 1996; 64 (6): 412–418.
Nyirenda MJ, Lindsay RS, Kenyon CJ, Burchell A, Seckl JR . Glucocorticoid exposure in late gestation permanently programs rat hepatic phosphoenolpyruvate carboxykinase and glucocorticoid receptor expression and causes glucose intolerance in adult offspring. J Clin Invest 1998; 101: 2174–2181.
Moss TJ, Sloboda DM, Gurrin LC, Harding R, Challis JRG, Newnham JP . Programming effects in sheep of prenatal growth restriction and glucocorticoid exposure. Am J Physiol Regul Integr Comp Physiol 2001; 281 (3): R960–R970.
Bloom SL, Sheffield JS, Mcintire DD, Leveno KJ . Antenatal dexamethasone and decreased birth weight. Obstet Gynecol 2001; 97 (4): 485–490.
Thorp JA, Jones PG, Knox E, Clark RH . Does antenatal corticosteroid therapy affect birth weight and head circumference? Obstet Gynecol 2002; 99 (1): 101–108.
French N, Hagan R, Evans SF, Godfrey M, Newnham J . Repeated antenatal corticosteroids: size at birth and subsequent development. Am J Obstet Gynecol 1999; 180: 114–121.
Crowther CA, Haslam RR, Hiller JE, Doyle LW, Robinson JS . Neonatal respiratory distress syndrome after repeat exposure to antenatal corticosteroids: a randomised controlled trial. The Lancet 2006; 367: 1913–1919.
Thorp JA, Jones AM, Hunt C, Clark R . The effect of multidose antenatal betamethasone on maternal and infant outcomes. Am J Obstet Gynecol 2001; 184 (2): 196–202.
Smith GCS, Smith MFS, McNay MB, Fleming JEE . First-trimester growth and the risk of low birth weight. N Eng J Med 1998; 339 (25): 1817–1822.
Newnham JP, Moss TJ . Antenatal glucocorticoids and growth: single versus multiple doses in animal and human studies. Semin Neonatol 2001; 6 (4): 285–292.
Piazze J, Berretta AR, Cioccio D, Anceschi M . Neonatal length and cranial circumference are reduced in human pregnancies at term after antepartum administration of betamethasone. J Perinat Med 2005; 33 (5): 463–464.
Oken E, Kleinman K, Rich-Edwards J, Gillman M . A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr 2003; 3 (6): 1–10.
Hadlock F, Harris R, Sharman R, Deter R, Park S . Estimation of fetal weight with the use of head, body and femur measurements—a prospective study. Am J Obstet Gynecol 1985; 151: 333–337.
Jobe AH, Newnham JP, Moss TJ, Ikegami M . Differential effects of maternal betamethasone and cortisol on lung maturation and growth in fetal sheep. Am J Obstet Gynecol 2003; 188: 22–28.
Wapner RJ, Sorokin Y, Thom EA, Johnson F, Dudley DJ, Spong CY et al. Single versus weekly courses of antenatal corticosteroids: Evaluation of safety and efficacy. Am J Obstet Gynecol 2006; 3 (195): 633–642.
Dalziel SR, Walker NK, Parag V, Mantell C, Rea HH, Rodgers A et al. Cardiovascular risk factors after antenatal exposure to betamethasone: 30-year follow-up of a randomised controlled trial. Lancet 2005; 365: 1856–1862.
Shelton SD, Boggess KA, Murtha AP, Groff AO, Herbert WN . Repeated fetal betamethasone treatment and birth weight and head circumference. Obstet Gynecol 2001; 97 (2): 301–304.
Murphy VE, Smith R, Giles WB, Clifton VL . Endocrine regulation of human fetal growth: the role of the mother, placenta, and fetus. Endocr Rev 2006; 27 (2): 141–169.
Drake AJ, Tang JI, Nyirenda MJ . Mechanisms underlying the role of glucocorticoids in the early life programming of adult disease. Clin Sci 2007; 113 (10): 219–232.
Rose JC, Schwartz J, Green J, Kerr DR . Development of the corticotropin-releasing factor adrenocorticotropic hormone/Beta-endorphin system in the mammalian fetus. In: Polin RA, Fox WW (eds). Fetal and Neonatal Physiology. W. B. Saunders: Philadelphia, USA, 1998, pp 2431–2442.
Winter SDW . Fetal and neonatal adrenocortical physiology, in Fetal and Neonatal Physiology In: Polin RA, Fox WW (eds). W. B. Saunders: Philadelphia, 1998, pp 378–403.
Mesiano S, Jaffe RB . Developmental and functional biology of the primate fetal adrenal cortex. Endocr Rev 1997; 18 (18): 378–403.
Austin MP, Leader L . Maternal stress and obstetric and infant outcomes: epidemiological findings and neuroendocrine mechanisms. Aust N Z J Obstet Gynaecol 2000; 40 (3): 331–337.
Albiston AL, Obeyesekere VR, Smith RE, Krozowski ZS . Cloning and tissue distribution of the human 11β-hydroxysteroid dehydrogenase type 2 enzyme. Mol Cell Endo 1994; 105 (2): R11–R17.
Brown RW, Kotolevtsev Y, Leckie C, Lindsay RS, Lyons V, Murad P et al. Isolation and cloning of human placental 11β-hydroxysteroid dehydrogenase-2 cDNA. Biochem J 1996; 313: 1007–1017.
Ballard PL, Ballard RA . Scientific basis and therapeutic regimens for use of antenatal glucocorticoids. Am J Obstet Gynecol 1995; 173: 254–262.
Sloboda DM, Challis JRG, Moss TJ, Newnham JP . Synthetic glucocorticoids: antenatal administration and long-term implications. Curr Pharm Des 2005; 11 (11): 1459–1472.
Acknowledgements
This research was supported by grants from the NIH (HD-50662 & NS-41298).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Davis, E., Waffarn, F., Uy, C. et al. Effect of prenatal glucocorticoid treatment on size at birth among infants born at term gestation. J Perinatol 29, 731–737 (2009). https://doi.org/10.1038/jp.2009.85
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jp.2009.85
Keywords
This article is cited by
-
Neurological implications of antenatal corticosteroids on late preterm and term infants: a scoping review
Pediatric Research (2022)
-
Optimizing antenatal corticosteroid therapy for improving outcome of premature infants
Pediatric Research (2019)
-
Contemporary Challenges and Developments: Antenatal Corticosteroid Therapy
Current Obstetrics and Gynecology Reports (2019)
-
Antenatal dexamethasone exposure differentially affects distinct cortical neural progenitor cells and triggers long-term changes in murine cerebral architecture and behavior
Translational Psychiatry (2017)
-
Association between gestational age at birth, antenatal corticosteroids, and outcomes at 5 years: multiple courses of antenatal corticosteroids for preterm birth study at 5 years of age (MACS-5)
BMC Pregnancy and Childbirth (2014)