Abstract
Staphylococcus aureus is one of the most commonly isolated microbes in chronic rhinosinusitis (CRS) that can be complicated due to the formation of a staphylococcal biofilm. In this study, we investigated antimicrobial efficacy of single mupirocin and three types of monoterpenes (thymol, menthol and 1,8-cineole) as well as mupirocin–monoterpene combinations against S. aureus ATCC 29213 and 5 methicilin-resistant S. aureus strains (MRSA) grown in planktonic and biofilm form. MIC against planktonic bacteria as well as minimum biofilm-eliminating concentrations (MBECs) and minimum biofilm inhibitory concentrations (MBICs) were determined by TTC and MTT reduction assay, respectively. The MICs of mupirocin (0.125–0.156 μg ml−1) were three orders of magnitude lower than the MICs of monoterpenes, which were as follows: thymol (0.250–0.375 mg ml−1) > menthol (1 mg ml−1) > 1,8-cineole (4–8 mg ml−1). Mupirocin-monoterpene combinations showed indifferent effect as compared with MICs of single substances. Mupirocin (0.016–2 mg ml−1) failed to destroy the biofilm. The MBECs of thymol and menthol were two- to sixfold higher than their MICs, while 1,8-cineole exerted a weak antibiofilm effect with MBECs 16- to 64-fold higher than MICs. Mixture of mupirocin and 1,8 cineole exerted a potentiated biofilm-eliminating effect, mupirocin–menthol showed antagonism, while effect of thymol–mupirocin mixture was inconclusive. MBICs of antimicrobials were close to their MICs, except 1,8-cineole, MBIC was about three- to fivefold higher. Dominant synergy was observed for mixtures of mupirocin and menthol or thymol, whereas mupirocin-1,8-cineol exerted an indifferent or additive biofilm inhibitory effect. Particular combinations of mupirocin and the monoterpenes could be applied in CRS therapy in order to eliminate or prevent bacterial biofilm growth.
Similar content being viewed by others
Introduction
Staphylococcus aureus is one of the most important human colonizers and pathogens that causes a variety of diseases, from mild skin and soft tissue infections to more serious conditions including pneumonia, endocarditis, osteomyelitis and sepsis.1 Also, it is one of the most commonly isolated microbes in chronic rhinosinusitis (CRS), a chronic disease of the paranasal sinuses that affects a large number of individuals in the developed world.2, 3, 4 Apart from the methicilin resistance of S. aureus (MRSA), infections with S. aureus can even further complicate owing to biofilm formation. In CRS, mucosal changes result in favorable conditions for the development of a S. aureus biofilm. Once a biofilm is established, its resistance to both host defences and antimicrobials (restricted penetration, decrease in bacterial metabolism and growth rate, increase in antibiotic-degrading enzymes accumulation and enhancement of exchanging rate of genes encoding for resistance) leads to chronic inflammation.5, 6, 7, 8 Mupirocin or pseudomonic acid A is natural antibiotic produced by Pseudomonas fluorescens, which has been successfully used as topical agent in the treatment of S. aureus-associated wound infections. It is a structural analog of isoleucyl-adenylate (Ile-AMP), which competes with Ile-AMP for binding sites of the isoleucyl-tRNA synthetases leading to abrogation of protein synthesis in bacteria.9, 10 Recently, Bode et al.11 showed that the nasal decolonization of S. aureus carriers by mupirocin significantly reduces the risk of S. aureus infections after surgery. However, data on the efficacy of mupirocin treatment in biofilm-associated nasal S. aureus infections as well as mupirocin antibiofilm activity in vitro are scarce. The development of antibiotic resistance in pathogenic bacteria increased general interest in studying the antimicrobial potency of phytochemicals including monoterpenes menthol, thymol and 1,8-cineole. These amphipathic phytochemicals with prevalent hydrophobicity evoke alterations of S. aureus membrane permeability causing leakage of intracellular material.12 The studies undertaken so far have shown that some phytochemicals including thymol might have impact on bacterial biofilm formation and development, acting as control agents of bacterial adhesion and cell-to-cell communication known as quorum sensing-QS.12 However, the biofilm eradication ability of such phytochemicals is insufficiently investigated. Taking into account the amphipathic nature of menthol, thymol and 1,8-cineole, these monoterpenes could penetrate through the exopolysaccharide matrix of S. aureus biofilm and enhance the activity of mupirocin trough membrane permeability disturbances. Therefore, the aim of our study was to investigate the antimicrobial efficacy of mupirocin alone and the mentioned monoterpenes as well as mupirocin–monoterpene combinations against S. aureus planktonic and biofilm modes of growth in vitro.
Materials and Methods
Bacterial strains
Staphylococcus aureus ATCC 29213 and clinical MRSA isolates (n=5) were used in this study. All of the strains (MFBF 10674, MFBF 10676, MFBF 10677, MFBF 10679, MFBF 10680) were taken from the microbe culture collection of Department of Microbiology, Faculty of Pharmacy and Biochemistry, University of Zagreb.
Antimicrobials
Mupirocin calcium dihydrate was donated by Pliva (Pliva, Zagreb, Croatia), whereas monoterpenes l-menthol (Sigma, St Louis, MO, USA), thymol (Kemika, Zagreb, Croatia) and 1,8-cineole (Merck, Darmstadt, Germany) were purchased.
Immediately before the assay, mupirocin calcium dihydrate was dissolved and diluted in an appropriate broth. Monoterpenes were dissolved in 100% ethanol (Kemika, Zagreb, Croatia) and diluted in an appropriate broth. Stock solution of Tween 80 in sterile water (5%) was used for homogenization of monoterpene alcoholic solutions.
Determination of MIC against planktonic bacteria
A stock culture of S. aureus strains was prepared, grown at 37 °C aerobically for 24 h in Müller–Hinton broth (Merck, Germany). A 1.5 × 108 colony-forming unit (c.f.u.) per ml inoculum was prepared using a nephelometer (BioMereiux, Marcy-l'Etoile, France) and adjusted to a final concentration 5 × 105 c.f.u. ml−1 in Müller–Hinton broth containing antimicrobials. The determination of MICs for mupirocin, menthol, thymol and 1,8-cineole were carried out using a twofold microdilution method, according to Clinical and Laboratory Standards Institute guidelines.13 Concentrations of twofold serial-diluted solutions in Müller–Hinton broth used for determining MIC for mupirocin were in a range from 0.0625 to 128 μg ml−1, concentrations of menthol, thymol and 1,8-cineole were in ranges of 0.03125–64 mg ml−1, 0.125–128 mg ml−1 and 0.125–128 mg ml−1, respectively. Also, a determination of MIC for combinations of mupirocin and each of monoterpenes was carried out. Concentrations of twofold serial-diluted solutions used for assessing MIC of combinations were in ranges of 0.015625–0.5 μg ml−1 (mupirocin), 0.5–4 mg ml−1 (menthol), 0.125–8 mg ml−1 (thymol) and 0.125–32 mg ml−1 (1,8-cineole). Concentration gradients were decreased in the same direction and the MICs of both compounds were in the same well. Culture of S. aureus in the broth with 0.31–1.25% of ethanol and 0.016–0.063% tween was used as positive (growth) control, whereas broth without S. aureus as negative control. Plates were incubated for 24 h, at 37 °C in aerobic atmosphere. At the end of incubation, 2,3,5-triphenyltetrazolium chloride (TTC, Sigma) was added as a visualization agent to the final concentration of 0.5 mg ml−1. Surviving bacteria metabolize the TTC and produce red formazan. MIC was derived from a microtitre plate as the minimum concentration of antimicrobial agent in the unstained wells. Examination for every strain was derived in quadruplicate. Mean concentration was used as a final result.
To simplify interpretation of MIC for combinations, Fractional Inhibitory Concentration Index (FICI) was calculated using an equation (Equation (1)). The combination of two antimicrobial agents is consider to be synergistic when FICI is less or equal to 0.5, additive when it is >0.5 and ⩽1, indifferent when it is >1 and ⩾4.0, and antagonistic when it is >4 (cited in ref. 14).

Biofilm formation
Biofilm was formed according to the method by Walencka et al.15 with minor modifications. Stock inoculum was prepared as described previously and adjusted to 1,5 × 106 c.f.u. ml−1 in Tryptic soy broth (TSB, Difco, Le Pont de Claix, France), supplemented with 0,25% d-(+)-glucose (Kemika, Zagreb, Croatia; TSBGlc). Diluted bacterial suspensions were incubated for 24 h at 37 °C, aerobically.
Following incubation, the suspension was diluted 20-fold with TSBGlc. A total of 100 μl of this suspension was added to each well of 96-well tissue culture plate (Nuncoln Surface, Nunc, Thermo Fisher Scientific, Waltham, MA, USA) and incubated for 24 h at 37 °C aerobically, to form the biofilm. To visualize a biofilm, a method by Kairo et al.16 was used, with a few modifications. After biofilm formation, wells were emptied and gently rinsed with 100 μl of saline, to remove planktonic bacteria and preserve the biofilm. After rinsing, 50 μl of MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, MTT reagent, Sigma) was added into each well and incubated for 2 h at 37 °C. MTT is metabolized by living bacterial cells forming purple formazan crystals. The formazan crystals were dissolved by adding 150 μl of dimethyl sulfoxide (DMSO, Sigma). The plate was shaken for 10 min until all formazan crystals dissolved. The absorbance was measured on a microplate reader (iEMS, Labsystems) at wavelength of 540 nm.
Determination of minimum biofilm-eliminating concentration
The biofilm of each S. aureus strain was grown on the bottom of wells of a 96-well microtiter plate as described above. Initial biofilm was stained and its absorbance was measured as described above. The obtained absorbance was defined as the absorbance of the initial biofilm, Aib.
In other wells, the intact initial biofilm was treated by adding 100 μl of antimicrobial agent solution. Concentration ranges of antimicrobials were 0.016 μg ml−1–2 mg ml−1 (mupirocin), 0.5–64 mg ml−1 (menthol), 0.25–32 mg ml−1 (thymol) and 2–256 mg ml−1 (1,8-cineole). Concentrations were prepared by diluting stock solutions with Tryptic soy broth (TSB, Merck, Germany). Control strains were grown in broth with and without 5–10% of ethanol + tween. Plates were incubated for 24 h at 37 °C aerobically. Following incubation, the wells were emptied and rinsed, and the biofilm remaining on the bottom of the wells was stained and measured as described previously. The obtained absorbance was defined as the absorbance of surviving biofilm, Asb. Antimicrobial treatment of each strain as well as controls was derived in quadruplicate. Viability was calculated using Aib and Asb by Equation (2). (Minimum biofilm-eliminating concentration (MBEC) is defined as minimum concentration of antimicrobial agent solution in well whose viability is ⩽10%).

According to Chou,17 results were plotted into corresponding linear form of median-effect plot  and using linear regression, MBEC was determined as a concentration where viability was 10% (Equation (3)).
and using linear regression, MBEC was determined as a concentration where viability was 10% (Equation (3)).

Mupirocin was combined with each monoterpene separately. The method is similar to the determination of MIC for combinations—the concentration gradient went in the same direction. A total of 50 μl of mupirocin solution was added into each well, and 50 μl of monoterpene solution to final concentration ranges of 0.016–0.125 μg ml−1 (mupirocin), 0.5–4 mg ml−1 (menthol), 0.125–1 mg ml−1 (thymol) and 16–128 mg ml−1 (1,8-cineole). Concentrations were prepared by diluting stock solutions with TSB. Plates were incubated for 24 h at 37 °C in aerobic atmosphere. Controls were grown in the same manner as described previously. After incubation, the biofilm remained on the bottom of the wells was visualized as described earlier. The obtained absorbance was defined as absorbance of survived biofilm (Asb). Using absorbances of initial and survived biofilm, viability and MBEC were calculated by the abovementioned equations (Equations (2) and (3)). All experiments were performed in quadruplicate.
Determination of minimum biofilm inhibitory concentration
Bacterial inoculums were prepared and adjusted to a concentration of 1.5 × 106 c.f.u. ml−1 in TSBGlc as described previously. Upon 24 h of incubation at 37 °C, bacterial suspensions were 10-fold diluted in TSBGlc.
A total of 50 μl of antimicrobial agent was added to the wells of 96-well plates in final concentration ranges of 0.016–1 μg ml−1 (mupirocin), 0.5–16 mg ml−1 (menthol), 0.063–4 mg ml−1 (thymol) and 4–128 mg ml−1 (1,8-cineole). The concentrations were prepared by diluting stock solutions with TSBGlc. Controls were treated with 0.63–5% of ethanol in TSBGlc. After achieving a concentration gradient, 50 μl of prepared bacterial suspensions were added to each well. The plates were incubated aerobically for 24 h at 37 °C. Upon incubation, biofilm was visualized as described previously.
Viability was calculated using the absorbance of biofilm formed by untreated bacteria (Aut) and absorbances of biofilm in treated bacteria (At) according to Equation (4).

MBIC is defined similarly as MBEC—using linear regression after plotting results into corresponding linear form of median-effect plot and calculating the concentration needed for viability of 10% (Equation (3)).
The MBIC of mupirocin−monoterpene combinations were determined as follows. A total of 25 μl of mupirocin solution was added to the wells to set a final concentration range of 0.002–0.125 μg ml−1 in combination with thymol and 1,8-cineole, and final concentration range of 0.0005–0.016 mg ml−1 in combination with menthol. Afterwards, 25 μl of monoterpene solution was added to wells in final concentration ranges of 0.125–1 mg ml−1 (menthol), 0.016–1 mg ml−1 (thymol) and 1–64 mg ml−1 (1,8-cineole). The concentrations were prepared by diluting stock solutions with TSBGlc. A total of 50 μl of bacterial suspension 10 times diluted in TSBGlc was added into each well. The plate was incubated for 24 h at 37 °C and viable biofilm was measured as described above. All experiments were performed in quadruplicate. Interactions were defined by FIC Index (Equation (1)), in terms of MBIC of mupirocin–monoterpene combination and MBIC of antimicrobial agent applied alone.
Statistics
Statistically significant differences between staphylococcal viability observed upon treatment with single monoterpene and monoterpene-mupirocin combination were analyzed by unpaired t-test. The level of P<0.05 was considered statistically significant.
Results
Antimicrobial activity against planktonic Staphylococci
MICs for mupirocin, menthol, thymol and 1,8-cineole against tested bacterial strains are presented in Table 1. Mupirocin showed the strongest antimicrobial activity; MICs (0.125–0.156 μg ml−1) were three orders of magnitude lower than the MICs of monoterpenes. Among the monoterpenes, thymol showed the most potent antimicrobial activity (0.250–0.375 mg ml−1), followed by menthol (1 mg ml−1) and 1,8-cineole (4–8 mg ml−1). According to the calculated FIC Index (Table 2), all of the tested combinations showed indifferent effect except for mupirocin and thymol combination tested against MRSA strains MFBF 10674, 10677 and 10680 where an additive effect was observed.
Biofilm-eliminating effect
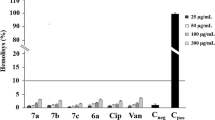
All S. aureus strains produced a solid biofilm on polystyrene microplates. Biofilms of all tested S. aureus strains showed resistance to mupirocin in all tested concentrations (0.016 μg ml−1–2 mg ml−1). Biofilm viability varied from 41.35 to 103.78% without any pattern through an increasing concentration of mupirocin (Figure 1). On the other hand, monoterpenes reduced biofilm viability down to 10%. Similar to MIC values, antibiofilm effect was best shown by thymol (BEC 0.59–1.07 mg ml−1), followed by menthol (BEC 3.21–6.35 mg ml−1) and 1,8-cineole (BEC>128 mg ml−1) (Table 1).
To test the biofilm eliminaton ability of mupirocin–monoterpene combinations, mupirocin was applied in the range of concentrations (0.016–0.125 μg ml−1) that reduced biofilm viability by approximately 10–60%, whereas monoterpenes were applied at concentration ranges that cover MBEC. The biofilm-eliminating effects of selected mupirocin–monoterpene combinations in comparison with monoterpene applied alone are presented in Figures 2, 3 and 4. Combination of menthol (4 mg ml−1) and mupirocin (0.125 μg ml−1) showed antagonism; single menthol-reduced biofilm by 90%, whereas combined treatment eliminated between 40 and 80% of biofilm. Opposite to that, thymol (0.5 mg ml−1) and mupirocin (0.063 μg ml−1) exerted potentiated effect, but only to three bacterial strains (P<0.05), whereas 1,8-cineol (128 mg ml−1) in combination with mupirocin (0.125 μg ml−1) showed significant potentiated biofilm-eliminating effect against all tested strains (P<0.05) and biofilm viability dropped to ~10%.
Biofilm inhibitory effect
The MBICs of mupirocin and monoterpenes applied alone that inhibited biofilm formation of each S. aureus strain by 90% are presented in Table 1. Mupirocin inhibited biofilm formation in concentrations close to its MICs (0.114–0.411 μg ml−1). MBICs of menthol (0.64–1.98 mg ml−1) and thymol (0.33–0.59 mg ml−1) were also closely to their MIC values, while MBICs of 1,8-cineole were approximately three- to fivefold higher than MICs.
Applying mupirocin–monoterpene combinations and calculating FIC Index, a dominant synergy was observed for mixtures of mupirocin and menthol or thymol, whereas the muprirocin and 1,8-cineol combination exerted an indifferent or additive biofilm inhibitory effect (Table 3).
Discussion
The standard assay for testing the antimicrobial susceptibility of planktonic bacteria demonstrated that mupirocin and monoterpenes menthol, thymol and 1,8-cineole applied alone were effective against S. aureus strain ATCC 29213 as well as against MRSA strains. The bacterial strains did not show significant difference in susceptibility with respect to a single antimicrobial agent. Among the tested antimicrobials, mupirocin exerted the most potent activity and MIC values were in accordance with previous reports.18, 19, 20 The antimicrobial potency of monoterpenes against S. aureus was as follows; MICs thymol<MICs menthol<MICs 1,8-cineole, and corresponded to literature data.21, 22, 23, 24 Trombeta et al.22 proposed a mechanism of antibacterial action for phenolic terpene thymol and alcoholic terpene menthol. These lipophilic compounds possess detectable water solubility and could migrate across the aqueous extracellular medium and interact with membrane lipids resulting in alterations of membrane permeability and leakage of intracellular materials. In addition, monoterpene transfer into the bacteria and interaction with intracellular structures could not be excluded.22 Taking into account the structure of 1,8-cineole we can assume that this cyclic ether monoterpene might have a similar mechanism of antibacterial action as was proposed for thymol and menthol. On the other hand, mupirocin is a hydrophilic antibiotic that enters into a bacterial cell by passive diffusion and interferes in protein synthesis by blocking the enzyme isoleucyl-tRNA synthetase.9, 10, 25 On the basis of the proposed antibacterial mechanisms we hypothesized that these compounds may act synergistically or at least additively. Surprisingly, mupirocin–monoterpene combinations showed a dominant indifferent effect against planktonic S. aureus strains.
Before we continue discussing the results on S. aureus biofilm susceptibility to mupirocin and monoterpenes, we would like to address the methodology for assessing the antibiofilm effects reported in the literature. Several colorimetric methods for biofilm susceptibility testing have been described for measuring active bacterial metabolism in biofilm,15, 26, 27, 28 or biofilm staining protocols29, 30 as well as turbidity measuring upon biofilm growth using Calgary Biofilm Device.31 Thus, a single standard method for biofilm susceptibility testing still does not exist as well as a univocal interpretation of results. This makes the comparison with already published results very difficult. In this study, the concentration that decreased bacterial viability, determined as metabolic activity in biofilm, to ⩽10% was defined as minimum biofilm-eliminating concentration (MBEC). Keeping in mind that antimicrobials affect mature biofilm, we may suggest that it is more appropriate to use the term MBEC rather than MBIC, which has been applied in some reports.15, 32 On the other hand, the term MBIC is more convenient to indicate the minimal concentration of an antimicrobial agent that inhibits the formation of biofilm. Such terminology has been used in several reports,26, 27, 28, 33 including the present study.
It is well known that bacteria in biofilm undergo phenotypic changes that make them several-fold more resistant to antimicrobials; for example, exopolysaccharide matrix may prevent the access of antibiotics to the bacteria, or cells within biofilm could have different growth characteristics and take up nutrients and drugs differently from planktonic bacteria.34, 35 Contrary to strong effect against planktonic S. aureus, mupirocin failed in destroying bacterial biofilm as determined by biofilm-eliminating MTT reduction assay. Low concentrations of mupirocin (0.016–0.125 μg ml−1) reduced biofilm by 10–60%, whereas an increase in concentration of up to 2 mg ml−1 did not have a significantly greater antibiofilm effect. Similar results were obtained by Hurler et al.26 who determined antibiofilm effects of mupirocin using resazurin reduction assay. When mupirocin was applied at a concentration of 0.5 μg ml−1, 40% of S. aureus biofilm reduction was observed, but biofilm eradication rate did not exceed 50% even with the highest concentration of antibiotic (0.2 mg ml−1). This could be due to the overproduction of exopolysaccharide which protects metabolically active bacteria embedded in the biofilm community and mupirocin could eliminate only the cells closest to the liquid-biofilm interface. Another study showed that mupirocin applied in low concentrations (7.81–125 μg ml−1) reduced 8-day-old S. aureus biofilm grown in cerebrospinal fluid broth by 90%.32 As was mentioned above, comparison is very difficult in this case owing to different methodologies.
Unlike mupirocin, thymol and menthol were able to reduce biofilm by 90% at concentrations two- to sixfold higher than their MICs. 1,8-cineole exerted a weak antibiofilm effect to all S. aureus strains with MBECs 16- to 64-fold higher than MICs. Contrary to biofilm eradication potency, all of the tested antimicrobials inhibited biofilm formation in concentrations close to their MICs, except 1,8-cineole whose MBIC was about three- to fivefold higher. The biofilm inhibitory properties of thymol are well documented, but the biofilm eradication potencies of all three monoterpenes are poorly investigated. Altogether, the antibiofilm potency of monoterpenes (thymol>menthol>1,8, cineole) against S. aureus presented in this study corresponds to available data on antibiofilm efficacy of these compounds against other Gram- positive and/or Gram-negative bacteria.24, 33, 36, 37, 38, 39 The relative hydrophilicity of monoterpenes may facilitate their penetration through the biofilm exopolysaccharide matrix, whereas their lipophilic nature enables interaction with bacterial membrane structures.39 Differences in their antibiofilm potency may be explained by their octanol-water partition coefficient, Po/w.40 It has been suggested that compounds with a log Po/w value higher than 3, such as thymol and menthol, are highly potent in disturbing a cell membrane, while 1,8 cineole with log Po/w 2.8 would exert weaker effect toward bacterial membrane structures.40, 41 In addition, menthol but not 1,8 cineole was able to increase the eucariotic membrane depolarization, which could be the reason behind the changes of ion flux across the membrane, the entry of Ca2+and loss of Cl− from cytoplasm.42 Monoterpenes applied in sub-inhibitory concentrations prior to biofilm formation could interact with staphylococcal surface proteins compromising initial attachment phase to polystyrene microtitre plates as well as interfering with the QS system.12, 33, 43
Considering the suggested mechanism of terpenoides antibiofilm action, we expected to observe potentiated effects of mupirocin–monoterpene combinations against mature staphylococcal biofilm. Indeed, 1,8-cineol in combination with mupirocin showed a potentiated biofilm-eliminating effect, whereas mupirocin–menthol mixtures exerted clear antagonism. We can only speculate that the simultaneous application of mupirocin and menthol resulted in the formation of some kind of complex with a exopolysaccharide matrix that could not disturb a mature biofilm significantly. For thymol–mupirocin combination biofilm eradication potency was inconclusive; the combination exerted potentiated biofilm-eliminating effect against three bacterial strains, to one strain the effect was indifferent and against two strains it was antagonistic. Opposite to the biofilm-eliminating effect, mupirocin–menthol as well as mupirocin–thymol showed dominant synergism in the inhibition of biofilm formation, whereas mupirocin-1,8 cineole combination exerted an indifferent or additive effect depending on the S. aureus strain. Monoterpenes probably inhibited Staphylococci adherence allowing mupirocin diffusion into bacteria.
When it comes to CRS, a variety of techniques, for example, scanning electron microscopy, transmission electron microscopy or confocal laser scanning microscopy, have revealed the presence of bacterial biofilms in the sinuses of patients.8 Prince et al.44 reported the reisolation of biofilm forming polymicrobial cultures of Pseudomonas aeruginosa, and/or S. aureus that comprised 71% of biofilm-positive CRS patients. Also, CRS patients infected with biofilm forming bacteria frequently develop chronic inflammation despite medical and surgical therapy.8 Our study demonstrated that mupirocin in combination with monoterpenes thymol, menthol or 1,8-cineole are more effective against staphylococcal biofilm than single compounds suggesting their usage in CRS therapy in order to prevent or eliminate bacterial biofilm growth. All of three monoterpenes has been traditionally used in treatment of various conditions and were proved to possess a various pharmacological activities including antimicrobial, anti-inflammatory, antioxidation etc. The effect of 1.8-cineole (200 mg per day) on arachidonic acid (AA) metabolism in blood monocytes of patients with bronchial asthma showed that this monoterpene could be used for tretamnet of bronchial astma.45 Menthol at concentration 0.1% (or 1 mg ml−1) in saline and 0.2% (2 mg ml−1) in petrolatum applied 3 times per day to the nasal passage of 16 subjects was not irritating to patients.46 Traditional uses of thyme include for coughs and upper respiratory congestion continues to be one of the most commonly recommended herbs for these indications. Standardized amounts of thyme oil may be found in commercial products, such as topical cosmetic formulations or mouthwash. Standardized extracts may contain 0.6–1.2% volatile oil and 0.5% (or 5 mg ml−1) thymol content.47
These data on application of monoterpenes in humans indicate that 1,8-cineole, thymol or menthol could be safely applied on nasal mucous particulary in concentrations that showed potentiated biofilm-eliminating activity (1,8-cineole) or sinergystic biofilm inhibition (menthol and thymol) when combined with low concentrations of mupirocin. Taking into account monoterpene anti-inflammatory properties,48 treatment with mupirocin–monoterpene combinations could have dual benefit reducing both biofilm growth and inflammation.
References
Reiß, S. et al. Global analysis of the Staphylococcus aureus response to mupirocin. Antimicrob. Agents Chemother. 56, 787–804 (2012).
Brook, I. The role of bacteria in chronic rhinosinusitis. Otolaryngologic Clinics of North America 38, 1171–1192 (2005).
Lin, A. & Busaba, N. Y. Staphylococcus aureus and endoscopic sinus surgery. Curr. Opin. Otolaryngol. Head Neck Surg. 14, 19–22 (2006).
Kilty, S. J. & Desrosiers, M. Y. The role of bacterial biofilms and the pathophysiology of chronic rhinosinusitis. Curr. Allergy Asthma Rep. 8, 227–233 (2008).
Hall-Stoodley, L. et al. Bacterial biofilms: from the natural environment to infectious diseases. Nat. Rev. Microbiol. 2, 95–108 (2004).
Lewis, K. Riddle of biofilm resistance. Antimicrob. Agents Chemother. 45, 999–1007 (2001).
Fux, C. A., Stoodley, P., Hall-Stoodley, L. & Costerton, J. W. Bacterial biofilms: a diagnostic and therapeutic challenge. Expert Rev. Anti. Infect. Ther. 1, 667–683 (2003).
Keir, J., Pedelty, L. & Swift, A. C. Biofilms in chronic rhinosinusitis: systematic review and suggestions for future research. J. Laryngol. Otol. 125, 331–337 (2011).
Hughes, J. & Mellows, G. Interaction of pseudomonic acid A with Escherichia coli B isoleucyl-tRNA synthetase. Biochem. J. 191, 209–219 (1980).
Thomas, C. M., Hothersall, J., Willis, C. L. & Simpson, T. J. Resistance to and synthesis of the antibiotic mupirocin. Nat. Rev. Microbiol. 8, 281–289 (2010).
Bode, L. G. M. et al. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N. Engl. J. Med. 362, 9–17 (2010).
Simões, M., Bennett, R. N. & Rosa, E. A. S. Understanding antimicrobial activities of phytochemicals against multidrug resistant bacteria and biofilms. Nat. Prod. Rep. 26, 746–757 (2009).
Nccls Performance Standards for Antimicrobial Susceptibility Testing. Clinical and Laboratory Standards Institute - NCCLS 27 (2007).
Mulyaningsih, S., Sporer, F., Zimmermann, S., Reichling, J. & Wink, M. Synergistic properties of the terpenoids aromadendrene and 1,8-cineole from the essential oil of eucalyptus globulus against antibiotic-susceptible and antibiotic-resistant pathogens. Phytomedicine 17, 1061–1066 (2010).
Walencka, E., Sadowska, B., Rózalska, S., Hryniewicz, W. & Rózalska, B. Lysostaphin as a potential therapeutic agent for staphylococcal biofilm eradication. Polish J. Microbiol 54, 191–200 (2005).
Kairo, S. K., Bedwell, J., Tyler, P. C., Carter, A. & Corbel, M. J. Development of a tetrazolium salt assay for rapid determination of viability of BCG vaccines. Vaccine 17, 2423–2428 (1999).
Chou, T.-C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 58, 621–681 (2006).
Sutherland, R. et al. Antibacterial activity of mupirocin (pseudomonic acid), a new antibiotic for topical use. Antimicrob. Agents Chemother. 27, 495–498 (1985).
Kresken, M., Hafner, D., Schmitz, F. J. & Wichelhaus, T. A. Prevalence of mupirocin resistance in clinical isolates of Staphylococcus aureus and Staphylococcus epidermidis: Results of the Antimicrobial Resistance Surveillance Study of the Paul-Ehrlich-Society for Chemotherapy, 2001. Int. J. Antimicrob. Agents 23, 577–581 (2004).
Dürrigl, M. et al. Spray dried microparticles for controlled delivery of mupirocin calcium: process-tailored modulation of drug release. J. Microencapsul. 28, 108–121 (2011).
Wattanasatcha, A., Rengpipat, S. & Wanichwecharungruang, S. Thymol nanospheres as an effective anti-bacterial agent. Int. J. Pharm. 434, 360–365 (2012).
Trombetta, D. et al. Mechanisms of antibacterial action of three monoterpenes mechanisms of antibacterial action of three monoterpenes. J. Antimicrob. Agents Chemother. 49, 2474–2478 (2005).
Guarda, A., Rubilar, J. F., Miltz, J. & Galotto, M. J. The antimicrobial activity of microencapsulated thymol and carvacrol. Int. J. Food Microbiol. 146, 144–150 (2011).
Hendry, E. R., Worthington, T., Conway, B. R. & Lambert, P. A. Antimicrobial efficacy of eucalyptus oil and 1,8-cineole alone and in combination with chlorhexidine digluconate against microorganisms grown in planktonic and biofilm cultures. J. Antimicrob. Chemother. 64, 1219–1225 (2009).
Capobianco, J. O., Doran, C. C. & Goldman, R. C. Mechanism of mupirocin transport into sensitive and resistant bacteria. Antimicrob. Agents Chemother. 33, 156–163 (1989).
Hurler, J., Sørensen, K. K., Fallarero, A., Vuorela, P. & Škalko-Basnet, N. Liposomes-in-hydrogel delivery system with mupirocin: In vitro antibiofilm studies and in vivo evaluation in mice burn model. Biomed. Res. Int. 2013, 498485 (2013).
Kwieciński, J., Eick, S. & Wójcik, K. Effects of tea tree (Melaleuca alternifolia) oil on Staphylococcus aureus in biofilms and stationary growth phase. Int. J. Antimicrob. Agents 33, 343–347 (2009).
Pettit, R. K. et al. Microplate alamar blue assay for Staphylococcus epidermidis biofilm susceptibility testing. Antimicrob. Agents Chemother. 49, 2612–2617 (2005).
Merritt, J. H., Kadouri, D. E. & O’Toole, G. A. Growing and analyzing static biofilms. Curr. Protoc. Microbiol (2005) Chapter 1: Unit 1B.1.
O’Toole, G. A. Microtiter dish biofilm formation assay. J. Vis. Exp. 47, 2437 (2011).
Ceri, H. et al. The Calgary Biofilm Device : new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms the calgary biofilm device : new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J. Clin. Microbiol. 37, 1771 (1999).
Ha, K. R., Psaltis, A. J., Butcher, A. R., Wormald, P.-J. & Tan, L. W. In vitro activity of mupirocin on clinical isolates of Staphylococcus aureus and its potential implications in chronic rhinosinusitis. Laryngoscope 118, 535–540 (2008).
Nostro, A. et al. Effects of oregano, carvacrol and thymol on Staphylococcus aureus and Staphylococcus epidermidis biofilms. J. Med. Microbiol. 56, 519–523 (2007).
Mah, T. F. C. & O’Toole, G. A. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 9, 34–39 (2001).
Sepandj, F., Ceri, H., Gibb, A., Read, R. & Olson, M. Minimum inhibitory concentration versus minimum biofilm eliminating concentration in evaluation of antibiotic sensitivity of enterococci causing peritonitis [1]. Perit. Dial. Int. 27, 464–465 (2007).
Braga, P. C., Dal Sasso, M., Culici, M. & Spallino, A. Inhibitory activity of thymol on native and mature Gardnerella vaginalis biofilms: in vitro study. Arzneimittelforschung 60, 675–681 (2010).
Husain, F. M. et al. Sub-MICs of Mentha piperita essential oil and menthol inhibits AHL mediated quorum sensing and biofilm of Gram-negative bacteria. Front. Microbiol. 6, 1–12 (2015).
Masadeh, M. M., Gharaibeh, S. F., Alzoubi, K. H., Al-Azzam, S. I. & Obeidat, W. M. Antimicrobial activity of common mouthwash solutions on multidrug-resistance bacterial biofilms. J. Clin. Med. Res. 5, 389–394 (2013).
El Abed, S. et al. Carvacrol and thymol components inhibiting Pseudomonas aeruginosa adherence and biofilm formation. Afr. J. Microbiol. Res. 5, 3229–3232 (2011).
Griffin, S., Wyllie, S. G. & Markham, J. Determination of octanol-water partition coefficient for terpenoids using reversed-phase high-performance liquid chromatography. J. Chromatogr. A 864, 221–228 (1999).
Weber, F. J. & De Bont, J. A. M. Adaptation mechanisms of microorganisms to the toxic effects of organic solvents on membranes. Biochim. Biophys. Acta. Rev. Biomembr 1286, 225–245 (1996).
Maffei, M., Camusso, W. & Sacco, S. Effect of Mentha x piperita essential oil and monoterpenes on cucumber root membrane potential. Phytochemistry 58, 703–707 (2001).
Knowles, J. R., Roller, S., Murray, D. B. & Naidu, A. S. Antimicrobial action of carvacrol at different stages of dual-species biofilm development by Staphylococcus aureus and Salmonella enterica serovar Typhimurium. Appl. Env. Microbiol 71, 797–803 (2005).
Prince, A. et al. Prevalence of biofilm-forming bacteria in chronic rhinosinusitis. Am. J. Rhinol. 22, 239–245 (2008).
Juergens, U. R., Stöber, M., Schmidt-Schilling, L., Kleuver, T. & Vetter, H. Antiinflammatory effects of euclyptol (1.8-cineole) in bronchial asthma: inhibition of arachidonic acid metabolism in human blood monocytes ex vivo. Eur. J. Med. Res. 3, 407–412 (1998).
Bhatia, S. P., McGinty, D., Letizia, C. S. & Api, A.,M. Fragrance material review on dihydro l-menthol. Food Chem. Toxicol. 46, S218–S223 (2008).
Basch, E., Basch, E., Bevins, A. & Sollars, D. Thyme (Thymus vulgaris L.), Thymol. J. Herb. Pharmacother. 4, 49–67 (2004).
De Cássia, Da Silveira, E., Sá, R., Andrade, L. N. & De Sousa, D. P. A review on anti-inflammatory activity of monoterpenes. Molecules 18, 1227–1254 (2013).
Acknowledgements
The authors are grateful to Pliva (Zagreb, Croatia) for providing the mupirocin. This work was financially supported by the University of Zagreb (Grant No.1126) and Ministry of Science, Education and Sports of the Republic of Croatia (Grant No. 006–0061117–1242).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Kifer, D., Mužinić, V. & Klarić, M. Antimicrobial potency of single and combined mupirocin and monoterpenes, thymol, menthol and 1,8-cineole against Staphylococcus aureus planktonic and biofilm growth. J Antibiot 69, 689–696 (2016). https://doi.org/10.1038/ja.2016.10
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ja.2016.10
This article is cited by
-
Synthesis and antimicrobial activity of new thiomonoterpene carboxylic acids
Russian Chemical Bulletin (2024)
-
High prevalence of mgrB-mediated colistin resistance among carbapenem-resistant Klebsiella pneumoniae is associated with biofilm formation, and can be overcome by colistin-EDTA combination therapy
Scientific Reports (2022)
-
Human organoid biofilm model for assessing antibiofilm activity of novel agents
npj Biofilms and Microbiomes (2021)
-
Formulation development of cream with mupirocin and essential oils for eradication of biofilm mediated antimicrobial resistance
Archives of Microbiology (2021)
-
Antibacterial effects of a polypeptide-enriched extract of Rana chensinensis via the regulation of energy metabolism
Molecular Biology Reports (2020)