Abstract
Objectives:
The corticotrophin-releasing factor (CRF)/urocortin system is expressed in the adipose tissue of mammals, but its functional role in this tissue remains unknown.
Methods:
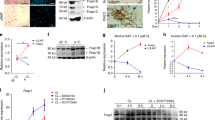
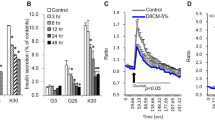
Pharmacological manipulation of the activity of CRF receptors, CRF1 and CRF2, was performed in 3T3L1 white pre-adipocytes and T37i brown pre-adipocytes during in vitro differentiation. The expression of genes of the CRF/urocortin system and of markers of white and brown adipocytes was evaluated along with mitochondrial biogenesis and cellular oxygen consumption. Metabolic evaluation of corticosterone-deficient or supplemented Crhr1-null (Crhr1−/−) mice and their wild-type controls was performed along with gene expression analysis carried out in white (WAT) and brown (BAT) adipose tissues.
Results:
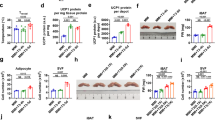
Peptides of the CRF/urocortin system and their cognate receptors were expressed in both pre-adipocyte cell lines. In vitro pharmacological studies showed an inhibition of the expression of the CRF2 pathway by the constitutive activity of the CRF1 pathway. Pharmacological activation of CRF2 and, to a lesser extent, inhibition of CRF1 signaling induced molecular and functional changes indicating transdifferentiation of white pre-adipocytes and differentiation of brown pre-adipocytes. Crhr1−/− mice showed increased expression of CRF2 and its agonist Urocortin 2 in adipocytes that was associated to brown conversion of WAT and activation of BAT. Crhr1−/− mice were resistant to diet-induced obesity and glucose intolerance. Restoring physiological circulating corticosterone levels abrogated molecular changes in adipocytes and the favorable phenotype of Crhr1−/− mice.
Conclusions:
Our findings suggest the importance of the CRF2 pathway in the control of adipocyte plasticity. Increased CRF2 activity in adipocytes induces browning of WAT, differentiation of BAT and is associated with a favorable metabolic phenotype in mice lacking CRF1. Circulating corticosterone represses CRF2 activity in adipocytes and may thus regulate adipocyte physiology through the modulation of the local CRF/urocortin system. Targeting CRF receptor signaling specifically in the adipose tissue may represent a novel approach to tackle obesity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Fekete EM, Zorrilla EP . Physiology, pharmacology, and therapeutic relevance of urocortins in mammals: ancient CRF paralogs. Front Neuroendocrinol 2007; 28: 1–27.
Carlin KM, Vale WW, Bale TL . Vital functions of corticotropin-releasing factor (CRF) pathways in maintenance and regulation of energy homeostasis. Proc Natl Acad Sci USA 2006; 103: 3462–3467.
Chen P, Van Hover C, Lindberg D, Li C . Central urocortin 3 and type 2 corticotrophin-releasing factor receptor in the regulation of energy homeostasis: critical involvement of the ventromedial hypothalamus. Front Endocrinol 2013; 3: 1–12.
Richard D, Lin Q, Timofeeva E . The corticotropin-releasing factor family of peptides and CRF receptors: their roles in the regulation of energy balance. Eur J Pharmacol 2002; 440: 189–197.
Zorrilla EP, Tache Y, Koob GF . Nibbling at CRF receptor control of feeding and gastrocolonic motility. Trends Pharmacol Sci 2003; 24: 421–427.
Tabarin A, Diz-Chaves Y, Consoli D, Monsaingeon M, Bale TL, Culler MD et alRole of the corticotropin-releasing factor receptor type 2 in the control of food intake in mice: a meal pattern analysis. Eur J Neurosci 2007; 26: 2303–2314.
Chen A, Brar B, Choi CS, Rousso D, Vaughan J, Kuperman Y et alUrocortin 2 modulates glucose utilization and insulin sensitivity in skeletal muscle. Proc Natl Acad Sci USA 2006; 103: 16580–16585.
Li C, Chen P, Vaughan J, Lee KF, Vale W . Urocortin 3 regulates glucose-stimulated insulin secretion and energy homeostasis. Proc Natl Acad Sci USA 2007; 104: 4206–4211.
Jamieson PM, Cleasby ME, Kuperman Y, Morton NM, Kelly PA, Brownstein DG et alUrocortin 3 transgenic mice exhibit a metabolically favourable phenotype resisting obesity and hyperglycaemia on a high-fat diet. Diabetologia 2011; 54: 2392–2403.
Boorse GC, Denver RJ . Widespread tissue distribution and diverse functions of corticotropin-releasing factor and related peptides. Gen Comp Endocrinol 2006; 146: 9–18.
Chen A, Blount A, Vaughan J, Brar B, Vale W . Urocortin II gene is highly expressed in mouse skin and skeletal muscle tissues: localization, basal expression in corticotropin-releasing factor receptor (CRFR) 1- and CRFR2-null mice, and regulation by glucocorticoids. Endocrinology 2004; 145: 2445–2457.
Seres J, Bornstein SR, Seres P, Willenberg HS, Schulte KM, Scherbaum WA et alCorticotropin-releasing hormone system in human adipose tissue. J Clin Endocrinol Metab 2004; 89: 965–970.
Sakamoto R, Matsubara E, Nomura M, Wang L, Kawahara Y, Yanase T et alRoles for corticotropin-releasing factor receptor type 1 in energy homeostasis in mice. Metabolism 2013; 62: 1739–1748.
Friedberg M, Zoumakis E, Hiroi N, Bader T, Chrousos GP, Hochberg Z . Modulation of 11 beta-hydroxysteroid dehydrogenase type 1 in mature human subcutaneous adipocytes by hypothalamic messengers. J Clin Endocrinol Metab 2003; 88: 385–393.
Smith GW, Aubry JM, Dellu F, Contarino A, Bilezikjian LM, Gold LH et alCorticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron 1998; 20: 1093–1102.
Grossi G, Bargossi AM, Lucarelli C, Paradisi R, Sprovieri C, Sprovieri G . Improvements in automated analysis of catecholamine and related metabolites in biological samples by column-switching high-performance liquid chromatography. J Chromatogr 1991; 541: 273–284.
Byrne HA, Tieszen KL, Hollis S, Dornan TL, New JP . Evaluation of an electrochemical sensor for measuring blood ketones. Diabetes Care 2000; 23: 500–503.
Ntambi JM, Young-Cheul K . Adipocyte differentiation and gene expression. J Nutr 2000; 130: 3122S–3126S.
Viengchareun S, Penfornis P, Zennaro MC, Lombes M . Mineralocorticoid and glucocorticoid receptors inhibit UCP expression and function in brown adipocytes. Am J Physiol Endocrinol Metab 2001; 280: E640–E649.
Harms M, Seale P . Brown and beige fat: development, function and therapeutic potential. Nat Med 2013; 19: 1252–1263.
Wu J, Cohen P, Spiegelman BM . Adaptive thermogenesis in adipocytes: is beige the new brown? Genes Dev 2013; 27: 234–250.
Seale P, Conroe HM, Estall J, Kajimura S, Frontini A, Ishibashi J et alPrdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest 2011; 121: 96–105.
Cannon B, Nedergaard J . Brown adipose tissue: function and physiological significance. Physiol Rev 2004; 84: 277–359.
Bartelt A, Heeren J . Adipose tissue browning and metabolic health. Nat Rev Endocrinol 2014; 10: 24–36.
Rosenwald M, Perdikari A, Rulicke T, Wolfrum C . Bi-directional interconversion of brite and white adipocytes. Nat Cell Biol 2013; 15: 659–667.
Bale TL, Picetti R, Contarino A, Koob GF, Vale WW, Lee KF . Mice deficient for both corticotropin-releasing factor receptor 1 (CRFR1) and CRFR2 have an impaired stress response and display sexually dichotomous anxiety-like behavior. J Neurosci 2002; 22: 193–199.
Yoshida-Hiroi M, Bradbury MJ, Eisenhofer G, Hiroi N, Vale WW, Novotny GE et alChromaffin cell function and structure is impaired in corticotropin-releasing hormone receptor type 1-null mice. Mol Psychiatry 2002; 7: 967–974.
Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S et alPRDM16 controls a brown fat/skeletal muscle switch. Nature 2008; 454: 961–967.
Barbatelli G, Murano I, Madsen L, Hao Q, Jimenez M, Kristiansen K et alThe emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am J Physiol Endocrinol Metabol 2010; 298: E1244–E1253.
Bale TL, Anderson KR, Roberts AJ, Lee KF, Nagy TR, Vale WW . Corticotropin-releasing factor receptor-2-deficient mice display abnormal homeostatic responses to challenges of increased dietary fat and cold. Endocrinology 2003; 144: 2580–2587.
Cullen MJ, Ling N, Foster AC, Pelleymounter MA . Urocortin, corticotropin releasing factor-2 receptors and energy balance. Endocrinology 2001; 142: 992–999.
Argiles JM, Fontes-Oliveira CC, Fuster G, Ametller E, Figueras M, Olivan M et alPatterns of gene expression in muscle and fat in tumor-bearing rats: effects of CRF2R agonist on cachexia. Muscle Nerve 2010; 42: 936–949.
Solinas G, Summermatter S, Mainieri D, Gubler M, Montani JP, Seydoux J et alCorticotropin-releasing hormone directly stimulates thermogenesis in skeletal muscle possibly through substrate cycling between de novo lipogenesis and lipid oxidation. Endocrinology 2006; 147: 31–38.
Timmons JA, Wennmalm K, Larsson O, Walden TB, Lassmann T, Petrovic N et alMyogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proc Natl Acad Sci USA 2007; 104: 4401–4406.
Tomlinson JJ, Boudreau A, Wu D, Atlas E, Hache RJ . Modulation of early human preadipocyte differentiation by glucocorticoids. Endocrinology 2006; 147: 5284–5293.
Chen A, Perrin M, Brar B, Li C, Jamieson P, Digruccio M et alMouse corticotropin-releasing factor receptor type 2alpha gene: isolation, distribution, pharmacological characterization and regulation by stress and glucocorticoids. Mol Endocrinol 2005; 19: 441–458.
Chen A, Vaughan J, Vale WW . Glucocorticoids regulate the expression of the mouse urocortin II gene: a putative connection between the corticotropin-releasing factor receptor pathways. Mol Endocrinol 2003; 17: 1622–1639.
Kershaw EE, Morton NM, Dhillon H, Ramage L, Seckl JR, Flier JS . Adipocyte-specific glucocorticoid inactivation protects against diet-induced obesity. Diabetes 2005; 54: 1023–1031.
Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T et alFunctional brown adipose tissue in healthy adults. N Engl J Med 2009; 360: 1518–1525.
Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH et alBeige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 2012; 150: 366–376.
Whittle A, Relat-Pardo J, Vidal-Puig A . Pharmacological strategies for targeting BAT thermogenesis. Trends Pharmacol Sci 2013; 34: 347–355.
Yoneshiro T, Aita S, Matsushita M, Kayahara T, Kameya T, Kawai Y et alRecruited brown adipose tissue as an antiobesity agent in humans. J Clin Invest 2013; 123: 3404–3408.
Bartelt A, Heeren J . The holy grail of metabolic disease: brown adipose tissue. Curr Opin Lipidol 2012; 23: 190–195.
Acknowledgements
Supported by INSERM (DC, AT), Region Aquitaine (DC, AT) and EquipEx OptoPath ANR-10-EQPX-08 (DC). We are indebted to Dr M Lombes (Institut de recherche Paris Sud, Le Kremlin-Bicêtre) for providing us with T37i cell line.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on International Journal of Obesity website
Rights and permissions
About this article
Cite this article
Lu, B., Diz-Chaves, Y., Markovic, D. et al. The corticotrophin-releasing factor/urocortin system regulates white fat browning in mice through paracrine mechanisms. Int J Obes 39, 408–417 (2015). https://doi.org/10.1038/ijo.2014.164
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2014.164