Abstract
Objectives:
To determine the effectiveness of a structured multidisciplinary non-surgical obesity therapy program on the basis of a temporary low-calorie-diet for 12 weeks, and additional intervention modules to enhance nutritional education, to increase physical activity and to modify eating behavior.
Design:
Prospective multicenter observational study in obese individuals undergoing a medically supervised outpatient-based 52-week treatment in 37 centers in Germany.
Subjects:
A total of 8296 participants with a body mass index (BMI) of >30 kg m−2 included within 8.5 years.
Measurements:
Main outcome measures were body weight loss, waist circumference (WC), blood pressure, quality of life and adverse events.
Results:
In females, initial body weight was reduced after the 1-year-intervention by 19.6 kg (95% confidence intervals 19.2–19.9 kg) and in males by 26.0 kg (25.2–26.8) according to per protocol analysis of 4850 individuals. Intention-to-treat (ITT) analysis revealed a weight reduction of 15.2 kg (14.9–15.6) in females and 19.4 kg (18.7–20.1) in males. Overall, the intervention resulted in mean reduction in WC of 11 cm; it reduced the prevalence of the metabolic syndrome by 50% and the frequency of hypertension from 47 to 29% of all participants (ITT, all P<0.001). The beneficial effects could be documented for up to 3 years and comprised significant improvement of health-related quality of life. The incidence of adverse effects was low; the only event repeatedly observed and possibly related to either the intervention or the underlying disease was biliary disorders.
Conclusion:
The present non-surgical intervention program is a highly effective treatment of obesity grades I–III and obesity-related diseases, and therefore, could be a valuable basis for future weight maintenance strategies required for sustained success.
Similar content being viewed by others
Introduction
Obesity is a chronic disease and a major health problem, with increases worldwide over a few decades at an alarming rate.1 European obesity prevalence ranges from 4.0–36.5%, depending on gender and geographical regions,2 approximating the prevalence of obesity among US adults, which has reached 31% in men and 33% in women.3 The consequences are enormous. Obesity is now recognized as the most important risk factor contributing to the health burden of the world, as it is associated with numerous complications, including type 2 diabetes, hypertension, cardiovascular disease, non-alcoholic fatty liver disease, arthritis, depression and, as shown more recently, certain cancers.4, 5 Obesity is estimated to reduce average life expectancy and is causing a major economic burden on health insurance.6, 7
This detrimental situation is worsened by the fact that the available obesity prevention and treatment tools are far from satisfactory. Either they lack validated effectiveness or they are not sufficiently established and accepted in the medical community, or they are invasive and side effects have not been thoroughly evaluated. Health insurances frequently refuse reimbursement of costs, which limits the availability of particular programs for large parts of the afflicted population. Two major reasons for insurers’ reluctance to pay for most programs include the insufficient scientific evidence for the efficiency of particular intervention measures and the cost-benefit justification of primary and additional ongoing costs for obesity treatment.
In the last few years, reports have shown that bariatric surgery is effective; however, the acceptance rate is unknown. Non-surgical weight-loss programs might have a higher acceptance rate, but have been poorly studied in a systematic manner or often failed to achieve and maintain desirable body weights.8 One of the few exceptions is the study by Wadden and Frey9 who showed that such a program is still effective after 5 years follow-up in 50% of participants who maintained medically significant weight loss of 5% or greater. In the present study, we aimed to further study the effectiveness of non-surgical approaches and analyzed a 52-week intervention program, which is medically supervised, out-patient-based and available in most major cities in Germany. Analysis of clinical data from all participants (>8000) over 8.5 years in 37 obesity centers in Germany shows that a multidisciplinary non-surgical weight loss program can be highly effective in reducing weight and weight-associated risk factors for up to 3 years.
Materials and methods
Intervention program
The present study was an investigator-initiated analysis of data from obese individuals who underwent a defined multidisciplinary non-surgical weight loss program (OPTIFAST52 (OF52) program, franchise holder Nestlé Inc., Vevey, CH).
The German OF52 program that covers 12 month of intervention was established in 1999. Data from all participants since 1999 until 2008 from 37 German centers were collected and included in an unselected manner. A previous 6-month-version had been launched a decade earlier in Germany, the US and other countries, and showed high initial success rates in selected centers over 6 months.8, 9 However, intention-to-treat (ITT) data regarding weight loss and reductions in comorbidities over 12 months from all centers conducting the program nationwide are not available from the 6-month-version. Only self-reported data sets from telephone interviews are available beyond 6 months.9 Therefore, we conducted a first comprehensive evaluation of treatment success of the German OF52 program after 12 months.
In contrast to the previous 6-month-version, the actual OF52 program contains an additional intensive weight loss maintenance training in the second half of the treatment year, focusing primarily on weight regain prevention and improvement of long-term success rates. Weight, waist circumference (WC), laboratory values, blood pressure and other clinical data were monitored regularly during the 52-week program. Only patients of OF52 were included in the present study. OF52 consists of a five-phase lifestyle modification program designed for 52 weeks, including meal replacement for 12 weeks and based on four modules (psychology, medicine, dietetics and exercise), imparted by a team of trained qualified health professionals such as psychologists, medical doctors, dietitians/nutritionists and physical therapists.10, 11, 12, 13, 14 During the program, closed groups of 8–15 persons meet weekly for about three and a half hours per session. The five program phases included (i) a 1-week-introduction time to check inclusion and exclusion criteria indicated below; (ii) a 12-week-period of low-calorie diet (LCD; 800 kcal per day) during which participants consume formula diet exclusively (daily consumption of five packets at 160 kcal each of meal replacement products dissolved in 300 ml water each; Optifast 800 formula, Nestlé Inc.), accompanied by 12 medical examinations, 12 exercise units, two behavior therapy lessons and two nutrition counselings; (iii) a 6-week-refeeding phase, during which solid food is reintroduced and formula diet is stepwise replaced by normal diet without change of total energy intake, accompanied by six medical examinations, six exercise units, two behavior therapy lessons and six nutrition counselings; (iv) a 7-week-stabilization phase in which energy intake is stepwise, enhanced to an individual level that allows weight stabilization, accompanied by three medical examinations, four exercise units, four behavior therapy lessons and three nutrition counselings; and (v) a 26-week-maintenance phase in which nutritional education and behavior modification is intensified to learn coping strategies and to achieve long-term weight control, accompanied by six medical examinations, 13 exercise units, 22 behavior therapy lessons and five nutrition counselings. The German OF52 uses a much more structured approach during the 52-week program than the 26-week Optifast-program in the US.9
Study population
At program start, all patients agreed in written form to provide their data anonymously. The inclusion criteria comprised an age 18–70 years, body mass index (BMI) >30 kg m−2, no comorbidities prohibiting participation in the program such as bedridden, cardiac or pulmonal insufficiency class III/IV according to the New York Heart Association, cardiac arrythmia, recent myocardial infarction, malignant disease, pregnancy or lactation, hypothyroidism, severe eating disorder and severe depression. Other comorbidities such as arterial hypertension, hypercholesterolemia, diabetes and other metabolic disorders were registered either by patient's history or medical records, or by abnormal laboratory findings. Arterial hypertension was defined as a systolic blood pressure >140 mm Hg or a diastolic blood pressure >90 mm Hg, hypertriglyceridemia as serum triglyceride levels >200 mg dl−1, hypercholesterolemia as serum low-density lipoprotein cholesterol levels >155 mg dl−1, fatty liver disease was assumed if alanine transaminase (ALT) was elevated (♀>35 ♂>50) in the absence of signs of liver disease of other origin and diabetes as pathological fasting serum glucose levels of >126 mg dl−1. Patients and family doctors were informed and recommended to treat the comorbidities according to guidelines; however, most participants had no drug treatment on a regular basis. Medical treatment was adapted during the program by the physician of the center in conjunction with the family doctors. All patients had to deliver a medical certificate for the necessity of treatment by their family doctor. Exclusion criteria included development of severe cardiopulmonary disease, malignant disease or pregnancy during participation.
Recruitment and data analysis
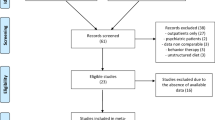
A defined data set was collected prospectively from each participant consisting of baseline data at start (T0), data of week 26 (T1) and at program termination at week 52 (T2). The data sets were collected mandatory in all German centers and transferred to a database, using special software provided by the program supplier (Optisoft; Nestlé Inc.). The 1-year-data were transmitted to an independent institute for data management (Campana and Schott, Frankfurt, Germany). The institute maintained anonymity of all patient data in the central database. During the 8.5 years evaluation period, 8894 matched the inclusion criteria, of which 8296 participants could be included into analysis (Figure 1).
Flow chart of study participants. Available data sets collected in 37 centers in Germany offering the program between July 1999 and December 2007. 1Two centers did not transfer their data due to data security reasons. 2Before starting analysis, the central database was purged of missing initial weight values and double entries. The 52-week-intervention was discontinued in 41.5% for different reasons such as personal reasons (6.9%), no further appearance (6.5%), job-related reasons (5.1%), disease/medical reasons (3.6%), financial reasons (3.4%), feeling of sufficient success (2.2%), familiar reasons (2.0%), mental/psychological reasons (1.5%), exclusion by the program team (1.4%), weight regain (1.0%), product dissatisfaction (0.6%) or pregnancy (0.4%). In about 1/6 of cases, the reason was unknown (7.2%).
Clinical parameters (age, sex, weight, height, WC, blood pressure) and laboratory parameters in serum (fasting glucose, triglycerides, total cholesterol/low-density lipoprotein–cholesterol, high-density lipoprotein cholesterol, ALT, γ-glutamyl-transpeptidase, sodium, potassium, creatinine and uric acid) were assessed. We calculated BMI, relative weight loss (RWL) in percent (100 × Δweight loss in kg/initial body weight in kg) and excess weight loss in percent (100 × Δweight loss in kg/initial body weight in kg − normal body weight in kg), whereas normal body weight was defined as the body weight corresponding to a BMI of 25 kg m−2. In all, 6759 out of 8296 participants’ (81%) WC was measured. In these patients, the presence of a metabolic syndrome (MS) was assessed according to the criteria of the International Diabetes Federation. As drug intake was not monitored consistently, only elevated laboratory parameters counted for the diagnosis of MS. The waist-to-height ratio (WHtR) was calculated according to the formula WHtR=WC/body height.
In addition, two subgroup analyses were performed with data derived from three centers. Data were pooled, as no significant differences in weight change were observed between the individual study centers. In the first subgroup (n=354), health-related quality of life (HRQOL) was assessed using the SF36 score.15 In a second subgroup of 301 out of 8296 participants (3.6%), long-term data on body weight could be obtained and analyzed 2 (T3) and 3 years (T4) after program start.
Statistical analysis
Values are presented as means and 95% confidence intervals (95% CI), if not indicated otherwise. Time courses were compared using the general linear model with repeated measures and contrasts for case differentiation. The unpaired two-samples t-test was employed for identification of baseline gender differences. Frequencies were compared using cross tables, according to the method of McNemar for time courses, and χ2-test for analysis of gender differences. P-values <0.001 were interpreted as statistically highly significant (***), P-values between 0.001 and <0.01 as very significant (**) and P-values between 0.01 and <0.05 as significant (*). Analysis of the main data set was performed using three approaches, the classical per-protocol analysis (PP) and ITT, and the recently recommended multiple imputation (MI) method in case of missing data, which allows the ability to impute plausible values for the missing data.16 However, despite some advantages of the MI for analysis of clinical trials, for example, on weight loss,17 we did not include thoroughly the MI analysis method in our results, because it yielded results being almost identical to those obtained by PP analysis. Obviously, employment of MI to our data set resulted in particular positive results (Figure 2), although literature suggests that the results would be between those derived from PP and ITT/last observation carried forward analysis. Moreover, according to sample size, the clinical trial is statistically overpowered and therefore does not necessarily demand imputation of missing data from participant ‘dropouts.’ In the ITT analysis, the final existent weight values of the dropouts were used according to the last observation carried forward method. All analyses were carried out by using the statistics software SPSS, version 17.0 (IBM SPSS, Chicago, IL, USA) and Graph Pad Prism, version 5 (GraphPad Software, Inc., La Jolla, CA, USA).
Mean weight changes of subjects participating in the 52-week-weight loss program. (a). PP analysis in 4850 participants, (b) MI analysis in 8296 participants, (c) ITT analysis in 8296 participants and (d) dropout analysis in 3446 participants, who did not complete the protocol. Percentages indicated at the right are RWLs at time points T2 or Tend, expressed in percent of body weight at program start (T0).
Results
Baseline characteristics
At study entry, 20% of the subjects emerged to have grade I obesity (BMI 30–34; 9 kg m−2), 32% grade II obesity (BMI 35–39.9) and 47% grade III obesity (BMI >40). Of the patients with obesity grade III, 10% had a BMI >50 and 2% >60. Arterial hypertension was present in 3157 subjects (38%), hypertriglyceridemia in 1684 (25%), hypercholesterolemia in 1168 (14%), elevated ALT in 793 (13%), pathological fasting glucose in 728 (11%) and elevated creatinine (♀>1, ♂>1,2 mg dl−1) in 406 (6%). Only 24% of the participants had no signs of obesity-associated diseases, according to this assessment. More than half of the participants fulfilled the MS criteria of the International Diabetes Federation. As expected, obesity was more pronounced in patients with MS (mean 122.9 kg, 95% CI, 122.1–123.7), compared with those without MS (mean 114.9 kg, 95% CI, 114.2–115.6; Δ1.5 BMI units, P<0.001). Patients with arterial hypertension, diabetes or elevated ALT values had a significantly higher BMI than those with normal range values (Δ2.7 BMI units, Δ2.9 BMI units and Δ1.2 BMI units, respectively; P<0.001). The baseline anthropometric and clinical data at study entry of the 8296 participants are summarized in Table 1. Of 8296 recruited participants, 3446 (42%) terminated the program before 52 weeks (Figure 1). Of those, 1818 (22% of all participants) dropped out within the first 26 weeks. Then, 1627 subjects (19%) dropped out after week 42. Reported reasons for dropout ahead of schedule were manifold (Figure 1).
Change in body weight and other anthropometric measures
The overall response rate to OF52 expressed as RWL calculated according to ITT analysis was 13.8% of the initial body weight. The highest response rate of 14.6% occurred in the subgroup of participants with an initial BMI of 40–50 kg m−2. According to PP analysis, an RWL of 20.4% (T1) and 17.9% (T2) of total body weight (P<0.001) was observed (Table 2 and Figure 2). Interestingly, even the preterm dropouts not included in the PP analysis underwent a significant weight reduction of in average 7%. In total, 82.1% of the completers and 64.3% of all participants successfully reduced initial body weight by 10% or more, corresponding to the WHO definition of successful weight loss. A weight reduction of at least 15% achieved 61.1% (PP analysis) and 47.6% (ITT analysis), respectively. Also the reduction in WC was highly significant (Table 2). Recently, the WHtR has been suggested as a better predictor of cardiovascular risk and mortality than WC in obese individuals.18 Therefore, we also calculated WHtR and found, based on ITT analysis, that the percentage of individuals with a normal WHtR <0.5 could be enhanced from 0.4–9.0% (P<0.001). Within the first 6 months of intervention (T0−T1), males were more successful than females in terms of weight reduction, but also in terms of reduction of BMI and WC (all P<0.001).
Change in blood pressure
Mean systolic blood pressure was reduced significantly from 141.1 mm Hg to 131.4 mm Hg (P<0.001), mean diastolic blood pressure was reduced from 88.2 mm Hg to 80.9 mm Hg (P<0.001) in subjects who completed the program (Figure 3). The ITT analysis yielded a reduction of systolic blood pressure by 8.0 mm Hg (P<0.001) and of diastolic blood pressure by 5.9 mm Hg (P<0.001). According to these data, more than 1000 individuals normalized their blood pressure in the course of the program (PP analysis, 1026 participants corresponding to 51% of the patients with hypertension at start and ITT analysis, 1137 participants corresponding to 36%). As only a minority of the participants received drug treatment at start (4%) and during the program (9%), either because they did not consult a doctor or they refused drug treatment, this effect is largely due to the weight reduction and life style change and not because of pharmacologic intervention.
Change in blood pressure in participants of a 52-week-weight loss program. Number of patients with hypertension (systolic >140 mm Hg or diastolic >90 mm Hg) and corresponding percentages based the whole study population are shown. (a) PP analysis, (b) ITT analysis. TEnd in the ITT analysis was calculated using the last observation carried forward method method. All percentages at time points T1, T2 and TEnd were significantly different compared with T0 (all P<0.001).
Change in laboratory data and occurrence of obesity-associated diseases
Within 1 year of OF52, values of key laboratory parameters were significantly reduced according to PP analysis, for example, triglycerides (from 166.1–133.0 mg dl−1), cholesterol (from 211.9–202.5 mg dl−1) and low-density lipoprotein–cholesterol (from 131.1–105.6 mg dl−1), respectively (all P<0.001). High-density lipoprotein–cholesterol increased within the year from 52.5–57.9 mg dl−1 (P<0.001). Levels of the liver enzyme ALT, which was elevated at program start in men and less pronounced in women, decreased significantly by 20% (P<0.001). Mean fasting glucose levels changed from 100.9–90.9 mg dl−1 (P<0.001). For uric acid, a mean reduction of 14% was observed (P<0.001). These changes resulted in a significant decrease in the number of individuals with MS and with obesity-associated diseases (Table 3). The predictive value of baseline parameters (age, sex, height, weight, BMI, WC, blood pressure, laboratory means) with regard to weight loss was evaluated; however, no differences for such parameters were found when comparing succeeders (defined as RWL >15% after 1 year) and others.
Adverse effects reporting
LCD programs have been shown to be effective; however, potential adverse events must be considered. Therefore, all reported adverse events were monitored and analyzed. Of 8296 participants, 96.2% reported no adverse events at all. Most registered adverse events reported by 315 participants, either related or unrelated to the intervention, were mild and transient. However, two cases of death (0.02% of all participants) were reported for which no clear statement with regard to a possible relation to the intervention program was made. The most common event during the invention were alopecia (46 subjects, 0.6%), possibly caused by micronutrient deficiency, and constipation (24 subjects; 0.3%), easily corrected by increased fluid and fiber intake. The third most common event, likely related to obesity, were biliary disorders such as colics, jaundice and cholecystitis (18 subjects; 0.2%) caused of bile stones or sludge. In 14 participants, a cholecystectomy was necessary. Questionably related to obesity or to the intervention program are malignancies such as breast cancer, lung cancer and colorectal cancer (4 subjects, 0.05%). All other events were mild and occurred in less than 0.1%; they were either unrelated to the program, or so rare that no conclusion could be made.
Long-term outcome data
In a subgroup of 301 subjects, long-term data on body weight beyond the intervention period of 1 year could be obtained and analyzed for up to 3 years (Figure 4). The baseline parameters of these patients (44.7 years old, BMI 40.1 kg m−2, RWL 19.2%, excess weight loss 46.9%, all means) matched the overall study population during the 1-year-intervention program. The data show that even 3 years after start of intervention, the extent of weight loss remained statistically still highly significant, despite a relative weight gain of 15.1% (95% CI, 12.8–17.4) 2 years after program termination. The mean weight loss after 3 years was 5.9 kg (95% CI, 3.7–8.1), RWL 4.2% of initial body weight (95% CI, 2.3–6.0).
Long-term weight curves over 3 years. The interdisciplinary weight loss program was terminated after 12 months. Means (±95% CI) of data obtained from three centers at program start (T0), at program end (T1) and 12 months (T2) and 24 months (T3) later, are shown. In total, 301 unselected data sets were analyzed for which T0, T2 and T4 data were available. Of these data courses, measurements at T3 were available in 114 cases. §T4 data ranged between 30–39 months (95% CI, mean 36.4 months). ***P<0.001.
Quality of life
HRQOL was assessed in 325 subjects using the SF36 questionnaire. At the end of the 1-year-intervention –program, all parameters indicating physical and psychological aspects of HRQOL improved significantly, compared with baseline data (Figure 5). Even 3 years after program start, most of those parameters, for example, physical functioning (+11.6%, P<0.001), general health (+7.3%, P<0.01), vitality (+4.7%, P<0.001) and mental health (+7.3%, P<0.001), still indicated significant ameliorations compared with initial values.
Long-term outcome of HRQOL in patients who participated in an interdisciplinary weight loss program (means±95% CI). (a). Physical aspect of health according to SF36. (b). Psychological aspect of health according to SF36. Time points of data collection were at T0 (month 0; n=354), T2 (month 12; n=272) and T4 (month 36±6 after program entry; n=250). Statistics *P<0.05, **P<0.01, ***P<0.001 when compared with program start (T0). German reference population (see SF36 manual).
Discussion
Here, we show in a large cohort of patients with grade I–III obesity that a non-surgical obesity therapy program performed in specialized centers under ambulatory conditions over 52 weeks is highly effective in reducing body weight and obesity-associated diseases after 12 month. According to the WHO definition of successful weight loss (>10% loss of initial body weight), the grand majority of participants (81% according to PP and 64% according to ITT analysis) were successful after 1 year of treatment. Of the successful people, 2/3 achieved a weight loss of >15%, independent of the type of statistical analysis, a result not documented before by other non-surgical intervention programs. Moreover, WC was substantially reduced (by 21 cm in males and 16 cm in females), which could be of major clinical relevance, because already a 3 cm reduction of WC results in significant improvement of cardiometabolic risk factors.19 Interestingly, the effects were more pronounced in male than in female participants. This gender difference is most likely due to the fact that (1) males entered the program with higher body weights and higher BMI and (2) an energy intake of 800 kcal per day means a more pronounced restriction for males than for females.10
The beneficial effects of the program on body weight were not restricted to particular ranges of initial body weight. Weight reduction was most pronounced in participants with an initial BMI of >40–50 kg m−2, but the effects were almost as good in patients with moderate obesity (BMI 30–40 kg m−2) and in patients with severe obesity (BMI >50 kg m−2) who are often referred for bariatric surgery. Not only anthropometric, but also other risk parameters defining the MS were strongly reduced. Of particular importance is the pronounced effect of the intervention on blood pressure, which confirms previous reports.20, 21 Among patients with hypertension at program entry, blood pressure was reduced to an extent similar to that achieved by pharmacological treatment.21 These data strongly suggest that a non-surgical obesity therapy program performed under medical supervision not only reduces risk of metabolic diseases, but also has substantial drug cost-saving effects.
An important strength of the study is the fact that we included all participants who started the program without selection during a 8.5-year-period of evaluation, which moderates the drawback because of lack of a randomized controlled study design for obvious technical and ethical reasons. Results might be biased if only patients from centers with above average therapeutic care are included in evaluations. This has also to be taken into account when comparing the results with bariatric surgery, as only a fraction of all operated patients are evaluated in studies. The relevance of our data is further strengthened by three additional findings: (1) a clear association between improvement of markers of physical health and HRQOL, (2) signs of a sustained benefit beyond the intervention period of 1 year and (3) no relevant adverse effects after a careful analysis of safety over 10 years of experience.
Most importantly, our data also show that the weight loss achieved after 6 months of treatment were stabilized in most cases until the end of the program. Minor weight gain within week 27–52 of treatment was approximately 3 kg. Rapid weight gain within weeks (‘yo-yo-effect’) was not observed. The question often asked is whether a non-invasive treatment can maintain its effect beyond the 1-year-treatment period. In the past, the expectations were high in this respect, although no defined intervention followed the 1-year-program. Interestingly, we found in a subgroup of 301 participants from whom long-term data were available for analysis, that weight loss based on the initial body weight is still statistically highly significant and clinically substantial after 3 years of observation.
Concerning safety, a non-surgical intervention is generally preferable to an invasive therapy, because it does not alter the integrity of the digestive tract and has no irreversible consequences. Indeed, the few adverse events we saw were thoroughly harmless and infrequent, except the risk of biliary disease among people who lose weight through LCD, because of the missing stimulus for gallbladder contraction. However, not only restriction diets, but also obesity per se may account for the enhanced risk of cholelithiasis and sludge,22, 23 promoted by an increased hepatic secretion of cholesterol.24 The risk rises linearly with increasing obesity25 and is particularly high in women with extreme obesity.26 Two cases of death (0.02%) and five cases of malignant diseases (0.05%) occured within the year of intervention among 8296 participants. Taken into consideration that obesity was found to be associated with enhanced cancer incidence27 and mortality,28 the two reported cases of death do not at all suggest an increased mortality related to the intervention program.
Our data revealed that OF52 is for now almost similarly effective as several invasive treatment options for obesity such as banding or vertical gastroplasty, as reported in the Swedish Obese Subjects study in which individuals with similar initial BMI were included.29, 30 In completers, the average excess weight loss after our non-surgical intervention was 53%, which is close to the overall weight loss of 61% reported in a meta-analysis about bariatric surgery.31 Only the more invasive gastric bypass seems to be clearly more effective than the non-surgical obesity therapy program tested here.
Most recently, the randomized controlled Louisiana Obese Subjects Study32 showed that a 24-month-non-surgical primary care practice program with LCD (890 kcal per day) for up to 12 weeks, meal replacements and choice of pharmacotherapy is highly effective in a primary care setting. However, although participants in our study were less obese than in LOSS, RWL at 1 year was higher after OF52 (17.9%), compared with LOSS (13.1%). The higher effectiveness we achieved could be because our program is more precisely structured and contains more intensive external therapeutic support, combined with regular exercise program and a complete full meal replacement for 12 weeks for all participants. Another primary care approach with similar results as reported in the LOSS study has been published from the UK some years earlier.33 Such data indicate that structured interdisciplinary programs performed in specialized centers might be more effective than primary care approaches.
Our data indicate that the OF52 program is more effective than primary care practice program within an observation time of 12 month. Compared with other medically supervised proprietary programs, the OF52 outcome data are comparable to what has been reported for other programs when looking at the 3–6 month data (RWL 20% here versus 15–25% in Tsai and Wadden8). However, OF52 is definitively more effective after 12 month (18% here versus 8–9% in Tsai and Wadden8). Interestingly, the advantage of OF52 seen after 12 month seem to disappear after 3 years (4.2% here versus 7% in Tsai and Wadden8), suggesting that without continuation of treatment, the effects will fade away with time. At present, it is unclear if this long-term weakness of non-surgical programs, and to some extent also of surgical interventions, is unavoidable or just a result of the lack of appropriate follow-up programs after the initially successful intervention.
The advantages of conservative programs (higher acceptance rate, low rates of adverse events and lower costs) are contrasted by reports suggesting higher relapse rates, compared with those after bariatric surgery. However, our data confirm previous reports showing that non-surgical interventions, even though they differed to the program we accomplished here, are still effective up to 5 years after start.9 Most importantly, the rate of weight loss achieved by using the LCD within the first weeks of the program seems to be not only highly motivating, but also the most effective non-surgical method of sustained weight loss.11 According to our preliminary long-term data, about 30% of the patients were successful regarding weight reduction over a time period of 3 years. Of them, 7% successfully maintain or even further reduce their weight after intervention without further support and 22% of the participants slowly regain weight, but still remain clearly below their initial weight. Self-reported physical activity, treatment adherence and consumption of meal replacements were identified as most relevant factors associated with success.34 Interestingly, even after surgical intervention, a certain weight gain is observed.29, 30 The relative weight gain 3 years after lowest recorded weight is about 5% in surgery studies and 15% in our study, indicating the necessity of effective weight maintenance programs that must follow all primary interventions, even those with 12 months initial duration. On the other hand, bariatric surgery, depending on the extent of malabsorption induced, is definitely less prone to the risk of weight regain compared with all non-surgical intervention programs. To which extent this major problem of even initially successful non-surgical programs like OF52 can be attenuated by appropriate follow-up interventions needs to be studied in future trials.
In conclusion, we demonstrated that defined non-surgical intervention programs such as the OF52 program could be highly effective in reducing body weight and risks of obesity-associated diseases. Therefore, we propose such an approach as promising choice for the primary treatment of obesity in adults and confirm it to be the first option before considering bariatric surgery.
References
Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser 2000; 894: 1–253, i–xii.
Berghofer A, Pischon T, Reinhold T, Apovian CM, Sharma AM, Willich SN . Obesity prevalence from a European perspective: a systematic review. BMC Public Health 2008; 8: 200.
Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM . Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 2006; 295: 1549–1555.
Haslam DW, James WP . Obesity. Lancet 2005; 366: 1197–1209.
Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M . Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 2008; 371: 569–578.
Wang Y, Beydoun M, Liang L, Caballero B, Kumanyika S . Will all Americans become overweight or obese? Estimating the progression and cost of the US obesity epidemic. Obesity (Silver Spring) 2008; 16: 2323–2330.
Müller-Riemenschneider F, Reinhold T, Berghofer A, Willich SN . Health-economic burden of obesity in Europe. Eur J Epidemiol 2008; 23: 499–509.
Tsai AG, Wadden TA . Systematic review: an evaluation of major commercial weight loss programs in the United States. Ann Intern Med 2005; 142: 56–66.
Wadden TA, Frey DL . A multicenter evaluation of a proprietary weight loss program for the treatment of marked obesity: a five-year follow-up. Int J Eat Disord 1997; 22: 203–212.
Tsai AG, Wadden TA . The evolution of very-low-calorie diets: an update and meta-analysis. Obesity (Silver Spring) 2006; 14: 1283–1293.
Saris WH . Very-low-calorie diets and sustained weight loss. Obes Res 2001; 9 (Suppl 4): 295S–301S.
Dixon JB, Dixon ME . Combined strategies in the management of obesity. Asia Pac J Clin Nutr 2006; 15 (Suppl): 63–9.
Norris SL, Zhang X, Avenell A, Gregg E, Brown TJ, Schmid CH et al. Long-term non-pharmacologic weight loss interventions for adults with type 2 diabetes. Cochrane Database Syst Rev 2005; 2: CD004095, doi:10.1002/14651858.CD004095.pub2.
Wadden TA, Butryn ML, Wilson C . Lifestyle modification for the management of obesity. Gastroenterology 2007; 132: 2226–2238.
Ware J, Kosinski M, Keller S . SF-36 physical and mental health summary scores. A user's manual. 1994.
Sinharay S, Stern HS, Russell D . The use of multiple imputation for the analysis of missing data. Psychol Methods 2001; 6: 317–329.
Elobeid MA, Padilla MA, McVie T, Thomas O, Brock DW, Musser B et al. Missing data in randomized clinical trials for weight loss: scope of the problem, state of the field, and performance of statistical methods. PLoS One 2009; 4: e6624.
Schneider HJ, Friedrich N, Klotsche J, Pieper L, Nauck M, John U et al. The predictive value of different measures of obesity for incident cardiovascular events and mortality. J Clin Endocrinol Metab 2010; 95: 1777–1785.
Balkau B, Picard P, Vol S, Fezeu L, Eschwege E . Consequences of change in waist circumference on cardiometabolic risk factors over 9 years: Data from an Epidemiological Study on the Insulin Resistance Syndrome (DESIR). Diabetes Care 2007; 30: 1901–1903.
Chuang SY, Chou P, Hsu PF, Cheng HM, Tsai ST, Lin IF et al. Presence and progression of abdominal obesity are predictors of future high blood pressure and hypertension. Am J Hypertens 2006; 19: 788–795.
Bramlage P, Hasford J . Blood pressure reduction, persistence and costs in the evaluation of hypertensive drug treatment—a review. Cardiovasc Diabetol 2009; 8: 18. (doi: 10.1186/1475-2840-8-18).
Friedman GD, Kannel WB, Dawber TR . The epidemiology of gallbladder disease: observations in the Framingham Study. J Chronic Dis 1966; 19: 273–292.
Kamrath RO, Plummer LJ, Sadur CN, Adler MA, Strader WJ, Young RL et al. Cholelithiasis in patients treated with a very-low-calorie diet. Am J Clin Nutr 1992; 56 (1 Suppl): 255S–257S.
Shaffer EA, Small DM . Biliary lipid secretion in cholesterol gallstone disease. The effect of cholecystectomy and obesity. J Clin Invest 1977; 59: 828–840.
Maclure KM, Hayes KC, Colditz GA, Stampfer MJ, Speizer FE, Willett WC . Weight, diet, and the risk of symptomatic gallstones in middle-aged women. N Engl J Med 1989; 321: 563–569.
Shaffer EA . Gallstone disease: Epidemiology of gallbladder stone disease. Best Pract Res Clin Gastroenterol 2006; 20: 981–996.
Pischon T, Nothlings U, Boeing H . Obesity and cancer. Proc Nutr Soc 2008; 67: 128–145.
Flegal KM, Graubard BI, Williamson DF, Gail MH . Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA 2007; 298: 2028–2037.
Sjostrom L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 2004; 351: 2683–2693.
Sjostrom L, Narbro K, Sjostrom CD, Karason K, Larsson B, Wedel H et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 2007; 357: 741–752.
Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K et al. Bariatric surgery: a systematic review and meta-analysis. JAMA 2004; 292: 1724–1737.
Ryan DH, Johnson WD, Myers VH, Prather TL, McGlone MM, Rood J et al. Nonsurgical weight loss for extreme obesity in primary care settings: results of the Louisiana Obese Subjects Study. Arch Intern Med 2010; 170: 146–154.
Gen Pract J . Evaluation of the counterweight programme for obesity management in primary care: a starting point for continuous improvement. Br J Gen Pract 2008; 58: 548–54.
Wadden TA, West DS, Neiberg RH, Wing RR, Ryan DH, Johnson KC et al. One-year weight losses in the Look AHEAD study: factors associated with success. Obesity (Silver Spring) 2009; 17: 713–722.
Acknowledgements
We thank all 37 OPTIFAST centers in Germany who participated in data input into the national database and provided and cared for study participants: OPTIFAST centers Arnsberg, Bad Füssing, Berlin I, II, III, Birkenwerder, Bochum, Duisburg, Frankfurt I, II, Gießen, Göttingen, Hagen, Hamburg II, Hamm, Hannover, Heidelberg, Kaiserslautern, Koblenz, Köln, Landshut, Lingen, Ludwigshafen, Magdeburg, Marl, München, Neuss, Nürnberg, Oberhausen, Osnabrück, Regensburg, Salzburg (Austria), Stadtoldendorf, Stuttgart, Wandsbek, Wilhelmsburg and Würzburg. This work was supported by the ‘Competence Network of Obesity,’ research group ‘Obesity and the GI tract,’ funded by the Federal Ministry of Education and Research, Germany (No. FKZ 01GI0843 to SCB) and by Nestlé HealthCare Nutrition GmbH, Munich, Germany, who is part of the ‘Competence Network of Obesity’. The sponsors had no influence in study design, analysis and interpretation of data, as well as in the writing of the report.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
All authors are engaged in Optifast centers. Apart from that the authors declare no conflict of interest.
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Bischoff, S., Damms-Machado, A., Betz, C. et al. Multicenter evaluation of an interdisciplinary 52-week weight loss program for obesity with regard to body weight, comorbidities and quality of life—a prospective study. Int J Obes 36, 614–624 (2012). https://doi.org/10.1038/ijo.2011.107
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2011.107
Keywords
This article is cited by
-
„Glucagon-like peptide‑1“(GLP‑1)-Rezeptor-Agonisten – die neuen Wunderwaffen in der Adipositastherapie?
Journal für Gastroenterologische und Hepatologische Erkrankungen (2023)
-
Was ist gesichert in der Therapie der Adipositas
Die Innere Medizin (2022)
-
Cesarean Delivery and Risk of Excess Weight Among Brazilian Preschool Children
Maternal and Child Health Journal (2022)
-
The PROMOTE study (High-protein and resistance-training combination in overweight and obesity) for short-term weight loss and long-term weight maintenance for Chinese people: a protocol for a pilot randomized controlled trial
Trials (2020)
-
Comparing the effectiveness of general dietary advice versus a very low energy diet in an obese outpatient population in Australia
Eating and Weight Disorders - Studies on Anorexia, Bulimia and Obesity (2019)