Abstract
The prevalence of Haldane’s rule suggests that sex chromosomes commonly have a key role in reproductive barriers and speciation. However, the majority of research on Haldane’s rule has been conducted in species with conventional sex determination systems (XY and ZW) and exceptions to the rule have been understudied. Here we test the role of X-linked incompatibilities in a rare exception to Haldane’s rule for female sterility in field cricket sister species (Teleogryllus oceanicus and T. commodus). Both have an XO sex determination system. Using three generations of crosses, we introgressed X chromosomes from each species onto different, mixed genomic backgrounds to test predictions about the fertility and viability of each cross type. We predicted that females with two different species X chromosomes would suffer reduced fertility and viability compared with females with two parental X chromosomes. However, we found no strong support for such X-linked incompatibilities. Our results preclude X–X incompatibilities and instead support an interchromosomal epistatic basis to hybrid female sterility. We discuss the broader implications of these findings, principally whether deviations from Haldane’s rule might be more prevalent in species without dimorphic sex chromosomes.
Similar content being viewed by others
Introduction
Haldane’s rule is one of very few generalizations in evolutionary biology. It predicts that in crosses between closely related species, if either sex of the offspring suffers disproportionate fitness costs, such as reduced fertility or viability, it will be the heterogametic sex (Haldane, 1922). It is a widespread phenomenon, observed across a broad range of taxa, irrespective of whether males or females are heterogametic (e.g., mammals, birds, reptiles, amphibians, fish, insects, nematodes and the plant genus Silene (Coyne and Orr, 2004; Brothers and Delph, 2010; Schilthuizen et al., 2011; Delph and Demuth, 2016). The pervasiveness of the rule indicates that sex chromosomes might commonly have a key role in the establishment of postzygotic reproductive barriers and by extension, speciation (Presgraves, 2008; Qvarnström and Bailey, 2009; Johnson and Lachance, 2012; Phillips and Edmands, 2012). However, the majority of research on Haldane’s rule has been conducted in species with conventional sex determination systems (e.g., XY and ZW). Exceptions to the rule, although rare, do occur but have been understudied (Turelli and Orr, 1995; Laurie, 1997; Malone and Michalak, 2008; Watson and Demuth, 2012; Fraïsse et al., 2016). Atypical sex determination systems and exceptions to Haldane’s rule provide unique opportunities to test the generality of proposed genetic explanations. Here, we test the importance of X-chromosome incompatibilities in a rare deviation from Haldane’s rule for female sterility, which occurs in both cross-directions, in an XO sex determination system.
The general consensus from published research is that Haldane’s rule results from a composite of evolutionary processes (Coyne and Orr, 2004). This is unsurprising considering that fertility and viability largely represent distinct functional pathways (Orr, 1993b; Wu and Davis, 1993). Three of the most consistent genetic theories proposed to explain the ubiquity of Haldane’s rule (which are not mutually exclusive) are the dominance theory, faster male theory and the faster X theory (Coyne and Orr, 2004). The dominance theory (Muller, 1942; Orr, 1993a; Turelli and Orr, 1995) proposes that the heterogametic sex suffers disproportionate fitness effects because all X (or Z)-linked loci involved in incompatible interactions with other loci are expressed. In contrast, the homogametic sex will only be affected by dominant or codominant incompatibilities as recessive X-linked incompatibility loci will be masked by the other X chromosome. Therefore, a key assumption of the dominance theory is that X-linked incompatibility loci contributing to the manifestation of Haldane’s rule should be predominantly recessive. The dominance theory appears to be the most common underlying cause of Haldane’s rule, as it has the most empirical support and can explain both sterility and inviability irrespective of which sex is heterogametic (Davies and Pomiankowski, 1995; Coyne and Orr, 2004). The faster male theory (Wu and Davis, 1993) suggests that hybrid sterility is more prevalent in heterogametic males because of sex differences in the rate of evolution of sterility loci arising from stronger sexual selection in males. In addition, spermatogenesis has been suggested to be especially prone to hybrid dysfunction (Wu and Davis, 1993; Presgraves, 2008; Malone and Michalak, 2008). There is good empirical support for the faster male theory from introgression experiments in mosquitoes (Presgraves and Orr, 1998) and Drosophila (Coyne and Orr, 2004; Masly and Presgraves, 2007), and gene expression studies in Drosophila (Michalak and Noor, 2003; Ranz et al., 2004). However, the faster male theory fails to explain Haldane’s rule in female heterogametic taxa, despite the fact that many groups such as Lepidoptera obey Haldane’s rule for sterility (Presgraves, 2002). The faster X theory copes with this because it argues that X chromosomes disproportionately accumulate hybrid incompatibilities, as recessive loci that increase fitness in the heterogametic sex would accumulate more readily on the X chromosome as they are immediately exposed to selection (Charlesworth et al., 1987). Such a pattern could partly reflect ascertainment bias from underestimating autosomal effects in backcross designs (Wu and Davis, 1993; Hollocher and Wu, 1996), although genome-wide introgression studies in Drosophila controlling for this potential bias have identified a higher density of hybrid male sterility factors on the X chromosome compared with the autosomes (Masly and Presgraves, 2007). The faster X theory favours the occurrence of Haldane’s rule in both male and female heterogametic species but has the weakest empirical support out of the three main theories. Overall, these prominent genetic models all predict that X-linked incompatibilities have a central role in Haldane’s rule.
Unusual sex determination systems and taxa that disobey Haldane’s rule provide important opportunities to test the generality of these genetic models, to identify less well-recognized processes and to disentangle their relative contributions to Haldane’s rule (Malone and Michalak, 2008; Koevoets and Beukeboom, 2009; Schilthuizen et al., 2011). Traditionally, species with XO systems have been understudied, and the species pairs that have been examined have been found to conform to Haldane’s rule (Virdee and Hewitt, 1992; Baird and Yen, 2000; Baird, 2002; Woodruff et al., 2010; Kozlowska et al., 2012). Recently, Caenorhabditis nematodes (XO sex determination system) have emerged as a useful system for studying postzygotic reproductive barriers. Hybridisation studies have revealed that some of the species pairs exhibit Haldane’s rule (Baird, 2002; Dey et al., 2014; Bundus et al., 2015). However, the diversity of reproductive modes, with many of the Caenorhabditis species pairs examined involving gonochoristic (male/female) (Dey et al., 2014) and androdioecious (male/ hermaphrodite) partners may make them difficult to compare with dioecious taxa. Although the three main genetic models should still apply in XO taxa, the absence of dimorphic sex chromosomes might reduce the likelihood that Haldane’s rule will manifest (Johnson, 2010). An obvious distinction is the absence of Y chromosomes, which have been found to have an important role in male sterility in some species of Drosophila but not others (Coyne, 1985; Turelli and Orr, 2000). Additionally, the potential for meiotic drive or genomic conflict, which have been argued to contribute to Haldane’s rule for sterility, might be reduced in taxa with monomorphic sex chromosomes (Coyne et al., 1991; Frank, 1991; Tao et al., 2001; Johnson, 2010; McDermott and Noor, 2010; Meiklejohn and Tao, 2010).
As with most Orthopterans, the two closely related Australian field cricket species (Teleogryllus oceanicus and T. commodus) have an XX–XO sex determination system, yet they provide an intriguing rare exception to Haldane’s rule for sterility (Hogan and Fontana, 1973). As males of this species are heterogametic (XO—they inherit a single X chromosome from their mother) and females are homogametic (XX—they inherit an X from each parent), Haldane’s rule predicts that hybrid males should suffer disproportionate negative fitness effects. However, early studies reported that reciprocal F1 hybrid females experienced disproportionate sterility compared with hybrid males (Hogan and Fontana, 1973). Reasons for this exception to Haldane’s rule are not clear. Neither the dominance theory nor faster male evolution are viable explanations for this case of sex-biased effects. Both T. oceanicus and T. commodus share the same diploid number of chromosomes (2n=26+XO, XX), but differ in the frequency of chiasmata and structural rearrangements, especially on the X (Fontana and Hogan, 1969; Hogan and Fontana, 1973). As a result of these differences, one possibility is that X–X interactions during alignment and crossing over might be disrupted, resulting in meiotic dysfunction and thus hybrid female sterility. Furthermore, the X chromosome accounts for a large proportion of these species’ genomes (ca. 30% in the diploid female: genome size is ca. 4.8 gb for a diploid female, 0.8 gb for a single X chromosome; K Klappert; unpublished data/personal communication), increasing the potential for X-linked incompatibilities. Hogan and Fontana (1973) reported that hybrid females had degenerate ovaries and laid few eggs, suggesting a combination of incompatibilities targeting both somatic and germline cells in the female reproductive system.
In this experiment, we tested whether interactions between X chromosomes might explain female sterility and inviability in T. commodus and T. oceanicus. We introgressed X chromosomes from either species onto recombinant autosomal backgrounds over three generations of crosses, and tested the fertility and viability of the different cross-types. We predicted that females inheriting two different X chromosomes on an averaged autosomal background (i.e., sharing a similar proportion of autosomal material from both species) would be less viable and suffer reduced fertility compared with females with two pure parental species X chromosomes.
Materials and methods
Maintenance and rearing
We established laboratory populations from the offspring of ca. 35 wild caught females from each of two allopatric Australian populations (T. commodus—near Moss Vale, NSW and T. oceanicus near Townsville, QLD, Australia). Colonies were bred in the lab for at least three generations before the experiment began. Stock crickets were housed in 16-L plastic boxes of ca. 80 individuals in a 25 °C temperature-controlled room on a 12:12 light–dark cycle. They were provided twice weekly with Burgess Excel 'Junior and Dwarf' rabbit food and cotton wool pads for drinking water and supplied with cardboard egg cartons for shelter.
Cross-design
The experimental design was similar across the three generations of crosses (Figure 1). Penultimate instar juveniles were separated into single-sex boxes to ensure virginity. For crosses, virgin adult males and females ca. 10–20 days past eclosion were paired together in smaller boxes (7 × 5 cm2). Approximately 20 pairs per cross-type were used (Figure 2).
Schematic of the cross-design. Letters below the crosses indicate X-chromosome compositions of the female offspring (e.g., ‘CO’, ‘CC’, etc.) (a) F1 reciprocal hybrids: Reciprocal interspecies crosses. (b) Backcross 1 (BC1): Reciprocal F1 hybrid females (i) and males (ii) backcrossed to both parental species. Female hybrid crosses are highlighted in grey as we did not expect any offspring. Striped X chromosomes represent interspecies X recombinants. Arrows indicate the key comparisons, in which females either share or differ in their X-chromosome complement. (c) Backcross 2 (BC2): BC1 females backcrossed to their maternal species. The arrows indicate group comparisons. (H) indicates an interspecies recombinant X. Control crosses, of pure species pairs, were also carried out for the F1 and BC2 generations but are omitted for clarity.
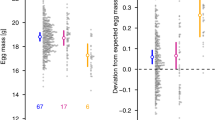
Three generations of crosses: (a) F1, (b) BC1 and (c) BC2, showing for each cross type: (i) numbers of eggs, (ii) numbers of offspring and (iii) proportion of female offspring (n=number of pairs per cross-type; n=20 for backcrosses with hybrid females). The X axis is labelled based on the female offspring XX type, the first letter reflects the maternal species X and the second letter the paternal species X (C=T. commodus; O=T. oceanicus). In BC1 and BC2, (H) indicates potential interspecies recombination on the X. Significant comparisons are highlighted by brackets (*P>0.01, **P>0.001, ***P<0.001). In the last row, error bars indicate 95% confidence intervals (binomial test) for the observed proportions, and dashed lines indicate a 50:50 sex ratio expectation.
Females oviposited in moist cotton pads; these egg pads were collected every 3–4 days and mating pairs were kept together for a 10-day period. Eggs were counted by examining the egg pads with a magnifying glass. The collected egg pads were monitored every 3–4 days, to prevent desiccation and to check for hatchlings. Newly hatched offsprings were provisioned with food and cardboard shelter. Egg pads were retained for 2–3 weeks and the final hatchling count was conducted ca. 3 weeks after the final egg pad was removed. Sex ratios were estimated once the hatchlings reached the penultimate instar juvenile stage (ca. 2 months), which is within days of adult sexual maturity.
In the first-generation crosses (F1), which comprised heterospecific and conspecific pairs, we investigated whether the species obey Haldane’s rule for inviability and whether unidirectional or bidirectional incompatibilities exist between them. The cross-types were classified by two letter codes, indicating the female offspring sex chromosome type. The first letter indicates the maternal species identity and the second the paternal species identity (C=T. commodus; O=T. oceanicus) (Figure 1). In the second-generation (BC1), reciprocal F1 hybrid females and males were backcrossed to both parental species to test whether the species obey Haldane’s rule for sterility and if X-linked incompatibilities contribute to offspring inviability. The key comparisons were between backcross types in which female offspring shared, on average, the same autosomal background (~75:25% species combination) but differed in their complement of X chromosomes (Figure 1b). We predicted that cross-types in which females inherited two different species Xs would produce fewer hatchlings and a higher proportion of males due to X-linked incompatibilities, compared with crosses in which females inherited two of the same species Xs. In the third-generation (BC2), female offspring from BC1 were backcrossed to their maternal species to test directly whether X-linked incompatibilities contribute to female sterility. The key comparisons were again between groups, which on average shared the same autosomal background (~87.5:12.5% species combination expected) but differed in their sex chromosome complement; either inheriting two pure species X chromosomes or one pure and the other an interspecies recombinant X (Figure 1c).
Statistical analysis
We used binomial tests to assess whether sex ratios differed from the predicted mean of 0.5 within each cross-type, and whether the sex ratios differed between the main groups of interest. Generalized linear models (GLMs) were fitted to test whether the X chromosome complement of females predicted their fertility, as would be expected if X-linked incompatibilities make a significant contribution to female fertility. All statistical analyses were performed in R (Version 3.1.3).
Our analyses focused on two types of data that reflect different processes: we compared the proportion of pairs exhibiting any response (a binary measure) among different cross-types, and we also examined differences in the magnitude of any responses (a continuous measure) among cross-types. For example, our response variables included (i) the proportion of pairs that produced eggs, (ii) the proportion that produced offspring, (iii) egg numbers, (iv) offspring numbers and (v) hatchling success rate (offspring/eggs). In each case, the main predictor of interest was female offspring XX type, which was fitted as a fixed effect. Female weight was fitted as a covariate. The decision to include or remove variables from models was made based on comparison of the model fit using analysis of variance and χ2 distributions (or F-test for quasi-likelihood models). Models were compared using the Akaike information criterion, and models with the lowest Akaike information criterion were considered the best fit.
The count data were heavily overdispersed (θ >20), thus we examined if quasi-binomial, quasi-poisson and negative binomial regression models fitted better using the 'MASS' package (Venables and Ripley, 2002). In some cases, the models were still overdispersed, thus zero-adjusted models were fitted. These allowed us to account for the excess of zeros and distinguish two different biological processes; whether females laid eggs, and if they did, how many hatched. There are two types of zero-adjusted models that differ in the treatment of zeros: zero inflated and zero altered (Zuur et al., 2012). Zeros in egg counts can be treated as arising from a single process, either females laid eggs or did not lay eggs, and therefore we used zero-altered models for egg counts (specifically zero-altered negative binomial (ZANB) models fitted best). The zero-altered negative binomial (ZANB) model uses two components, the positive (i.e., non-zero) data follows a truncated negative binomial distribution (negbin), whereas all the zero data is modelled together (binomial). However, an offspring count of zero could occur when females lay no eggs, or when females laid eggs but none hatched. Therefore, we used zero-inflated models for offspring counts (specifically ZINB models fitted best). Zero-inflated models assume there are two processes generating the zeros in the data and models these two processes separately, a poisson GLM for the count data and a binomial GLM for the occurrence of zeros. The package 'pscl' was used to fit zero-adjusted models (Zeileis et al., 2008). To test for differences between the groups of interest, Tukey's pairwise comparisons were fitted with the 'multcomp' package (glht function; Hothorn et al., 2008).
Results
F1 generation
Asymmetric production of F1 hybrids
Reproductive success was strongly asymmetric. Crosses between T. commodus females and T. oceanicus males (CO) had lower fertility compared with the reverse cross (OC) (Figure 2a and Table 1). Nearly all females laid eggs, but the number of eggs was markedly lower for CO crosses (mean±s.e.: CO, 84±27.75) compared with the reciprocal cross (OC, 239.56±34.28) (negative binomial GLM: Z3,80=−3.226, P=0.007; Table 1). There was an excess of zeros among CO pairs, as only 41% of CO pairs produced offspring compared with 70% for OC crosses (ZINB binomial: Z11,73=2.426, P=0.053). Females from the CO group also produced fewer offspring (mean±s.e., 55.73±21.8) than the OC cross (155.04±29.77), although this was nonsignificant (Table 1).
The asymmetry in reproductive success may be due to maternal effects or sperm–egg incompatibilities. If X-cytoplasmic interactions contribute to the asymmetry in F1 production, we predicted hybrid females would suffer disproportionate inviability compared to males as they inherit an X on a foreign species’ cytoplasmic background. However, the absence of sex-specific inviability indicates this is not the case (Figure 2a(iii)). In line with T. commodus females performing poorly when crossed to a heterospecific, they also had reduced fertility when paired with a conspecific partner in the F1 generation (Figure 2a(i–ii) and Supplementary Table S1). They produced both fewer eggs (parental CC vs parental OO: negative binomial GLM, Z3,80=2.374, P=0.082) and fewer offspring (ZINB negbin, Z3,80=−3.325, P<0.001). However, this species difference was not observed in the BC2 generation (Figure 2c(i–ii) and Supplementary Table S1).
No evidence of Haldane’s rule for inviability
All four F1 cross-types, two intraspecific (parental crosses) and two interspecific crosses, had a higher proportion of males than the expected 0.5 sex ratio (binomial exact test: P <0.001) (Figure 2a(iii)). Importantly, there was no differential viability between males and females in the hybrid crosses compared with the parental crosses (parental CC vs CO: X2=0.418, d.f.=1, P=0.518; parental OO vs OC: X2=0.02, d.f.=1, P=0.888). Therefore, there is no evidence for Haldane’s rule for inviability within these species.
BC1 generation
Reciprocal exceptions to Haldane’s rule for sterility
T. oceanicus and T. commodus provide a reciprocal exception to Haldane’s rule as nearly all hybrid females were sterile in both directions of the cross (only a single BC1 offspring was produced from 80 backcrosses), whereas all four hybrid male backcross types were fertile (Figure 2b(ii)). We predicted that hybrid male backcrosses that produced female offspring with heterospecific X chromosomes would exhibit reduced fertility (BC1: OO vs OC or CC vs CO) because of X–X interactions. We found no support for this hypothesis in either the proportion of pairs exhibiting a response or in the strength of response (i.e., number of eggs or offspring per pair) (Table 2). Contrary to the prediction that heterospecific X–X interactions would reduce fertility, CO pairs (T. commodus females paired with male hybrids carrying a T. oceanicus X chromosome) produced more eggs (mean±s.e.: CO 242.4±27.36) than the comparisons between CC pairs (T. commodus females paired with male hybrids carrying a T. commodus X chromosome) (mean±s.e.: 103.25±22.29) (ZANB negbin: Z9,70=3.72, P <0.001). However, the number of offspring was not significantly different between these two groups (mean±s.e.: CO 40.55±8.37 vs CC 22.5±6.89) (ZINB negbin: Z9,70=−0.861, P=0.389). In the other group comparison, there was no difference between OC and OO pairs in either the number of eggs or offspring (Table 2). The hatching success rate also did not differ among the groups of interest (Table 2). Overall, we detected no support for X–X interactions affecting fertility.
No X effect on viability
Under a scenario in which X-linked incompatibilities disproportionately affect viability, we predicted an excess of males due to female inviability in groups in which females inherited two different species Xs. Again, contrary to this prediction, there was a lower proportion of females in the OO group than the expected mean of 0.5 (Binomial exact test, P<0.001), and this was significantly lower than the comparison group OC (OO vs OC groups: X2=5.358, d.f.=1, P=0.021) (Figure 2b(iii)). Comparing the CC vs CO cross-types, there was no sex ratio bias (X2=2.326, d.f.=1, P=0.127). Overall, females that inherited two different species X chromosomes did not exhibit reduced viability.
BC2 generation
X–X interactions do not cause female sterility
We predicted that females with a mixed species complement of X chromosomes would suffer reduced fertility compared with females with conspecific X chromosomes. There was no difference between the CC vs (H)C groups in either the number of eggs produced (ZANB negbin: Z13,128=−0.418, P=0.992) or the number of offspring (ZINB negbin: Z13,128=0.417, P=0.991; Figure 2c(ii) and Supplementary Table S1). In line with our prediction, there was a marginal difference in fertility between OO vs (H)O groups. OO females appeared to produce more eggs (mean±s.e.: OO, 92.5±22.9 vs (H)O, 33.13±14.2), although this was not significant (ZANB negbin: Z13,128=−1.593, P=0.434; Table 3). However, OO pairs produced more offsprings than the corresponding (H)O group (mean±s.e.: OO, 28.68±10 vs (H)O, 6.92±3.34) (ZINB negbin: Z13,128=2.957, P=0.017; Table 3), which was consistent with our prediction that females with a mixed species complement of X chromosomes will suffer reduced fertility. Although the proportion of parental OO pairs (control crosses) that produced eggs was surprisingly low (0.56) (Supplementary Table S1), all parental pairs that produced eggs resulted in hatchlings, compared with a range of only 19–63% for the backcrosses.
Limited role for X chromosomes in inviability
Sex ratio data showed a higher proportion of females in the (H)O group compared with the OO group (Binomial test; X2=4.059, d.f.=1, P=0.044), indicating that (H)O males may suffer disproportionate inviability (Figure 2c(iii)). In this cross, males potentially inherit an interspecies recombinant X, which is hemizygous and could therefore expose them to an elevated likelihood of epistatic incompatibilities involving recessive X substitutions (e.g., X-autosomal incompatibilities). Comparisons between CC and (H)C revealed no significant sex ratio difference (Binomial test; X2=0.772, d.f.=1, P=0.38). Both parental species crosses showed a reduction of females from the expected mean of 0.5, particularly in the parental CC crosses (Figure 2c(iii)).
Discussion
Two important empirical findings in evolutionary biology, Haldane’s rule and the large X effect, are so consistent that they have been thought to be nearly universal (Coyne and Orr, 1989; Coyne and Orr, 2004). Both suggest that X chromosomes have a key role in the establishment of postzygotic barriers between species (Coyne and Orr, 1989; Masly and Presgraves, 2007; Presgraves, 2010; Johnson and Lachance, 2012; Phillips and Edmands, 2012). However, most research on the genetic basis of reproductive isolation has focused on male sterility and on male heterogametic species, as opposed to female fertility (though see Orr and Coyne, 1989; Davis et al., 1994; Hollocher and Wu, 1996; Watson and Demuth, 2012; Suzuki and Nachman, 2015). Rare cases in which homogametic females suffer disproportionate effects of hybridisation provide an important opportunity to investigate the genetic basis of female sterility and processes that may counter Haldane’s rule. Crosses between T. oceanicus and T. commodus provide one such remarkably rare exception to Haldane’s rule—female hybrids were almost uniformly sterile in our experiment, and out of 80 backcrosses with reciprocal hybrid females, only a single offspring hatched. A considerable number of hybrid females, derived from numerous different cross-types, produced eggs, indicating that not all ovaries are degenerate (Figure 2b(i)). This observation suggests a complex genetic basis for hybrid female sterility, in which certain hybrid genic combinations may occasionally result in fertile hybrid females in natural populations (Virdee and Hewitt, 1994).
Asymmetrical reproductive isolation
Asymmetrical genetic incompatibilities are a common observation among animal and plant hybridisations (Turelli and Moyle, 2007). They are believed to principally arise from negative epistasis between autosomal or sex-linked loci and uniparentally inherited maternal factors (e.g., mitochondrial DNA, cytoplasmic background) (Turelli and Orr, 2000; Turelli and Moyle, 2007; but see Bundus et al., 2015). We found a clear asymmetry in genetic compatibility. T. commodus females mated to T. oceanicus males produced far fewer eggs and offspring than the reciprocal cross (Figure 2a). In other words, hybridisation was more successful when the mother was T. oceanicus. This unidirectional incompatibility appears to manifest at a very early stage, as egg laying was disrupted.
Maternal effects (or cytonuclear incompatibilities) may lead to exceptions to Haldane’s rule for inviability if incompatibility loci are sex linked, as hybrid females inherit one of their X chromosomes on a different species’ cytoplasmic background. However, we did not detect any sex-specific inviability in comparisons between the F1 hybrid and parental species crosses (Figure 2a(iii)). Instead, sperm–egg incompatibilities or autosomal–cytoplasmic interactions, rather than X-cytoplasmic interactions, might be responsible for the asymmetrical reduction in fertility. If species differ in the degree of sperm competitiveness, asymmetric gametic isolation may occur (Martín-Coello et al., 2009). Females of both Teleogryllus species mate multiply in natural populations, and paternity is highly skewed, more so in T. oceanicus than T. commodus (Simmons and Beveridge, 2010). Heterospecific crosses with T. oceanicus males may therefore be predicted to have higher mating success compared with the reciprocal cross. However, this was not the case; heterospecific crosses with T. oceanicus males had reduced fertility compared with the reverse cross. Overall, Haldane’s rule does not manifest for any inviability patterns in crosses between these species.
Contrary to a previous report, which found a 1:1 sex ratio for pure-species crosses (Hogan and Fontana, 1973), we found a male biased sex ratio for both intra- and interspecific crosses. This discrepancy between the studies could have arisen due to population differences. The previous cytogenetic (Fontana and Hogan, 1969) and hybridisation work (Hogan and Fontana, 1973) was conducted on laboratory populations of T. oceanicus collected from Ayr, northern Queensland (ca. 90 km from where we sampled our study population in Townsville), and T. commodus from Melbourne, southern Victoria (ca. 750 km from where we sampled our study population in Moss Vale, New South Wales). In general, populations within a species can show a high degree of variation for genetic incompatibilities (Cutter, 2012) with other species, including X-chromosome inversions, endosymbiont strains or infection rates (e.g., Wolbachia (Telschow et al., 2005)) that alter sex ratios. However, the latter mechanisms usually result in female bias. In addition, differences in environmental conditions, such as temperature, or differential fertilization of nullo-X sperm may alter sex ratios (Wade et al., 1999; Bundus et al., 2015).
X-linked incompatibilities
What is the genetic cause of the deviation from Haldane’s rule for sterility in Australian Teleogryllus, and can it inform us more broadly about hybrid incompatibilities? Maternal effects (and cytonuclear incompatibilities) have previously been implicated in deviations from Haldane’s rule for inviability (Sawamura et al., 1993; Sawamura, 1996; Abe et al., 2005) but not sterility (Orr and Irving, 2001). Early developmental stages are predicted to be especially sensitive to maternal effects (Mousseau, 1991); however, little is known about maternal effects on adult reproductive traits. Disruption to early developmental stages could influence later reproductive output. However, we do not believe this explains hybrid female sterility in our study system, because maternal effects often exhibit asymmetrical effects and are not necessarily expected to influence both directions of the cross equally (Turelli and Moyle, 2007). Also, if maternal effects had a role in female sterility, we would predict backcrosses with hybrid males to be more compatible with their maternal species, which was not the case.
Laurie (1997) highlighted two factors that might promote exceptions to Haldane’s rule with respect to female hybrid sterility, and which affect both directions of a cross equally: X–X incompatibilities and dominant X-autosomal interactions. Both depend on X interactions, but our results yielded negligible support for the former. We hypothesized that reciprocal hybrid female sterility had a shared basis, namely due to chromosomal rather than genic interactions, in particular X–X interactions leading to meiotic dysfunction. Only one of our comparisons was consistent with X-linked incompatibilities reducing female fertility; a higher number of offspring produced from OO vs (H)O groups in BC2 (Figure 2c(ii) and Table 3). However, there was no detectable difference between the CC vs (H)C groups in BC2 (Figure 2c(ii) and Table 3). Furthermore, among the BC1 crosses the CO pairs produced more eggs on average than CC pairs (Figure 2b and Table 2). This pattern also refutes our prediction. If X–X incompatibilities were primarily responsible for the sterility of F1 hybrid females, we expected to observe a clear reduction in fertility for crosses in which females inherited two different X chromosomes. Instead, our results are more consistent with an epistatic origin of the incompatibilities due to Dobzhansky–Muller incompatibilities (Dobzhansky, 1937; Muller, 1942; Maheshwari and Barbash, 2011). This could be autosomal–autosomal or could still involve the X chromosome if these were dominant X–A interactions. We cannot unambiguously distinguish these, but the fact that there are large differences between similar genotypes that differ in the source of the X and A chromosomes, rather than the proportion of interspecies material (e.g., CC versus OO in BC1; Figure 2c), suggests that specific X–A interactions may contribute to lower female fertility.
The lack of a large X effect on female sterility might be explained by the fact that theory predicts a disproportionate accumulation of male but not female fertility loci on the X chromosome in male heterogametic species (Charlesworth et al., 1987). The loci underlying female fertility may be just as likely to accumulate on the autosomes as on the X (Masly and Presgraves, 2007), thus X-linked loci that affect male fertility would need to have pleiotropic effects in hybrid females to produce a large X effect on female fertility (Coyne and Orr, 1989; Presgraves, 2008). Introgression studies examining the large X effect in Drosophila have provided mixed results; some support the view that male and female sterility loci are qualitatively different (Wu and Davis, 1993; Coyne and Orr, 2004), whereas others have detected X effects on both male and female sterility (Orr, 1987; Orr and Coyne, 1989). In this study, we did not test the effect of X introgression on the fertility of both sexes, but the absence of evidence for a large X effect in females supports the view that X chromosomes do not have a pronounced role in female sterility.
XO sex determination system
As exceptions to Haldane’s rule are extremely rare, particularly in both directions of a cross, could deviations for female sterility be caused by a peculiarity of XO sex determination systems? While the main genetic models underlying Haldane’s rule should apply to XO systems, the absence of dimorphic sex chromosomes might relax the operation of some less well-recognized processes that could contribute to Haldane’s rule (e.g., meiotic drive, Y-incompatibilities). Previous hybridisation studies in XO taxa suggest they generally obey Haldane’s rule (Ohmachi and Masaki, 1964; Mantovani and Scali, 1992; Virdee and Hewitt, 1992; Baird and Yen, 2000; Baird, 2002; Woodruff et al., 2010; Kozlowska et al., 2012). However, only two previous reciprocal exceptions to Haldane’s rule have been described, one for inviability in an XO species (Spence, 1990) and the other for male sterility in a female heterogametic species (Malone and Michalak, 2008). The later exception can be explained under existing theory and has been experimentally shown to be due to faster male evolution (Malone and Michalak, 2008), which would not explain the exception to Haldane’s rule in our study system. The former case occurs in the Heteropteran pondskater Limnoporous spp., which has an XO sex determination system (Spence, 1990). Spence (1990) found that in crosses between Limnoporus notablis and L. dissortis, F1 hybrid females suffer disproportionate inviability compared with male hybrids. Applying a backcross design similar to that used in our study, Spence (1990) tested whether the presence of two different species X chromosomes contributed to hybrid inviability. However, his results differed from ours, because he detected a large X effect on female inviability. Considering that XO species represent a relatively small fraction of the species examined in hybridisation studies, yet exhibit two remarkably rare exceptions to Haldane’s rule (Limnoporous spp.—female inviability; Teleogryllus spp.—female sterility), future research would benefit from investigating why Haldane’s rule might be less prevalent in systems that lack dimorphic sex chromosomes.
Conclusions
T. commodus and T. oceanicus provide a rare exception to Haldane’s rule for sterility, but not inviability. Unexpectedly, we found negligible support for X-linked incompatibilities contributing to hybrid female sterility. This lack of support is surprising given the size of the X chromosomes in these species; when in single copy in males, the X chromosome represents ~20% of the diploid male genome, and when in two copies in females it represents ~30% of the diploid female genome (K Klappert; unpublished data/personal communication). Even though no large X effect was detected in our study, it does not rule out the potential for X-linked incompatibilities. However, the low fitness seen in backcross offspring, irrespective of their XX identity, suggests that partially dominant autosomal loci may supersede X-linked interactions in disrupting female fertility. Our results also revealed a clear asymmetry in fertility in reciprocal F1 crosses, with greater viability when hybrids were derived from T. oceanicus mothers, indicating that maternal effects (e.g., autosomal–cytoplasmic interactions) or sperm–egg incompatibilities might have an important role in reproductive barriers and asymmetric introgression between these species. Whether this rare exception to Haldane’s rule represents a more general pattern of deviation from the rule in systems without dimorphic sex chromosomes (e.g., XO systems, haplodiploid) remains to be determined.
References
Abe TA, Spence JR, Sperling FA . (2005). Mitochondrial introgression is restricted relative to nuclear markers in a water strider (Hemiptera: Gerridae) hybrid zone. Canadian Journal of Zoology 83: 432–444.
Baird SE . (2002). Haldane’s rule by sexual transformation in caenorhabditis. Genetics 161: 1349–1353.
Baird SE, Yen WC . (2000). Reproductive isolation in Caenorhabditis: terminal phenotypes of hybrid embryos. Evol Dev 2: 9–15.
Brothers AN, Delph LF . (2010). Haldane’s rule is extended to plants with sex chromosomes. Evolution 64: 3643–3648.
Bundus JD, Alaei R, Cutter AD . (2015). Gametic selection, developmental trajectories, and extrinsic heterogeneity in Haldane’s rule. Evolution 69: 2005–2017.
Charlesworth B, Coyne JA, Barton NH . (1987). The relative rates of evolution of sex chromosomes and autosomes. Am Naturalist 130: 113–146.
Coyne JA . (1985). The genetic basis of Haldane’s rule. Nature 314: 736–738.
Coyne JA, Charlesworth B, Orr HA . (1991). Haldane’s rule revisited. Evolution 45: 1710–1714.
Coyne JA, Orr HA . (1989). Two rules of speciation. In Endler J, Otte D eds Speciation and its Consequences. Sinauer: Sunderland, MA, USA. pp 180–207.
Coyne JA, Orr HA . (2004) Speciation. Sinauer Associates: Sunderland, MA, USA.
Cutter AD . (2012). The polymorphic prelude to Bateson–Dobzhansky–Muller incompatibilities. Trends Ecol Evol 27: 209–218.
Davies N, Pomiankowski A . (1995). Haldane’s rule: old theories are the best. Trends Ecol Evol 10: 350–351.
Davis AW, Noonburg EG, Wu C . (1994). Evidence for complex genic interactions between conspecific chromosomes in the Drosophila simulans Clade. Genetics 137: 191–199.
Delph LF, Demuth JP . (2016). Haldane’s rule: Genetic bases and their empirical support. J Hered 107: 383–391.
Dey A, Jin Q, Chen YC, Cutter AD . (2014). Gonad morphogenesis defects drive hybrid male sterility in asymmetric hybrid breakdown of Caenorhabditis nematodes. Evol Dev 16: 362–372.
Dobzhansky T . (1937) Genetics and the Origin of Species. Columbia University Press, New York, NY, USA.
Fontana PG, Hogan TW . (1969). Cytogenetic and Hybridisation studies of geographic populations of Teleogryllus commodus (Walker) and T. oceanicus (Le Guillou)(Orthoptera: Gryllidae). Austr J Zool 17: 13–35.
Fraïsse C, Gunnarsson PA, Roze D, Bierne N, Welch JJ . (2016). The genetics of speciation: Insights from Fisher's Geometric Model. Evolution 70: 1450–1464.
Frank SA . (1991). Haldane’s rule: a defense of the meiotic drive theory. Soc Study Evol 45: 1714–1717.
Haldane JBS . (1922). Sex ratio and unisexual sterility in hybrid animals. J Genet 12: 101–109.
Hogan TW, Fontana PG . (1973). Restoration of meiotic stability following artificial hybridisation and selection in Teleogryllus (Orth., Gryllidae). Bull Entomol Res 62: 557–563.
Hollocher H, Wu CI . (1996). The genetics of reproductive isolation in the Drosophila simulans clade: X vs autosomal effects and male vs female effects. Genetics 143: 1243–1255.
Hothorn T, Bretz F, Westfall P . (2008). Simultaneous inference in general parametric models. Biomet J 3: 346–363.
Johnson NA . (2010). Hybrid incompatibility genes : remnants of a genomic battlefield? Trends Genet 26: 317–325.
Johnson NA, Lachance J . (2012). The genetics of sex chromosomes: evolution and implications for hybrid incompatibility. Ann NY Acad Sci 1256: E1–22.
Koevoets T, Beukeboom LW . (2009). Genetics of postzygotic isolation and Haldane’s rule in haplodiploids. Heredity 102: 16–23.
Kozlowska JL, Ahmad AR, Jahesh E, Cutter AD . (2012). Genetic variation for postzygotic reproductive isolation between caenorhabditis briggsae and caenorhabditis sp. 9. Evolution 66: 1180–1195.
Laurie CC . (1997). The weaker sex is heterogametic: 75 years of Haldane’s rule. Genetics 154: 1419–1426.
Maheshwari S, Barbash DA . (2011). The genetics of hybrid incompatibilities. Annu Rev Genet 45: 331–355.
Malone JH, Michalak P . (2008). Physiological sex predicts hybrid sterility regardless of genotype. Science 319: 2008.
Mantovani B, Scali V . (1992). Hybridogenesis and androgenesis in the stick-insect Bacillus rossius–Grandii benazzii. Evolution 46: 783–796.
Martín-Coello J, Benavent-Corai J, Roldan ERS, Gomendio M . (2009). Sperm competition promotes asymmetries in reproductive barriers between closely related species. Evolution 63: 613–623.
Masly JP, Presgraves DC . (2007). High-resolution genome-wide dissection of the two rules of speciation in Drosophila. PLoS Biol 5: 1890–1898.
McDermott SR, Noor MaF . (2010). The role of meiotic drive in hybrid male sterility. Philos Trans R Soc Lond Ser B 365: 1265–1272.
Meiklejohn CD, Tao Y . (2010). Genetic conflict and sex chromosome evolution. Trends Ecol Evol 25: 215–223.
Michalak P, Noor MAF . (2003). Genome-wide patterns of expression in Drosophila pure species and hybrid males. Mol Biol Evol 20: 1070–1076.
Mousseau T . (1991). Maternal effects in insect life histories. Annu Rev Entomol 36: 511–534.
Muller HJ . (1942). Isolating mechanisms, evolution, and temperature. Biol Symp 6: 71–125.
Ohmachi F, Masaki S . (1964). Interspecific crossing and development of hybrids between the Japanese species of Teleogryllus (Orthoptera: Gryllidae). Soc Study Evol 18: 405–416.
Orr HA . (1987). Genetics of male and female sterility in hybrids of Drosophila pseudoobscura and D. persimilis. Genetics 116: 555–563.
Orr HA . (1993a). A mathematical model of Haldane’s rule. Evolution 47: 1606–1611.
Orr HA . (1993b). Haldane’s rule has multiple genetic causes. Nature 361: 532–533.
Orr HA, Coyne JA . (1989). The genetics of postzygotic isolation in the Drosophila virilis group. Genetics 121: 527–537.
Orr HA, Irving S . (2001). Complex epistasis and the genetic basis of hybrid sterility in the Drosophila pseudoobscura Bogota-USA hybridisation. Genetics 158: 1089–1100.
Phillips BC, Edmands S . (2012). Does the speciation clock tick more slowly in the absence of heteromorphic sex chromosomes? BioEssays 34: 166–169.
Presgraves DC . (2002). Patterns of postzygotic isolation in Lepidoptera. Int J Org Evol 56: 1168–1183.
Presgraves DC . (2008). Sex chromosomes and speciation in Drosophila. Trends Genet 24: 336–343.
Presgraves DC . (2010). Darwin and the origin of interspecific genetic incompatibilities. Am Naturalist 176: S45–S60.
Presgraves DC, Orr HA . (1998). Haldane’s rule in taxa lacking a hemizygous X. Science (New York, NY) 282: 952–954.
Qvarnström A, Bailey RI . (2009). Speciation through evolution of sex-linked genes. Heredity 102: 4–15.
Ranz JM, Namgyal K, Gibson G, Hartl DL . (2004). Anomalies in the expression profile of interspecific hybrids of Drosophila melanogaster and D. simulans. Genome Res 14: 373–379.
Sawamura K . (1996). Maternal effect as a cause of exceptions for Haldane’s rule. Genetics 143: 609.
Sawamura K, Taira T, Watanabe TK . (1993). Hybrid lethal systems in the Drosophila melanogaster species complex. I. The maternal hybrid rescue (mhr) gene of Drosophila simulans. Genetics 305: 299–305.
Schilthuizen M, Giesbers M, Beukeboom LW . (2011). Haldane ’ s rule in the 21st century. Heredity 107: 95–102.
Simmons LW, Beveridge M . (2010). The strength of postcopulatory sexual selection within natural populations of field crickets. Behav Ecol 21: 1179–1185.
Spence JR . (1990). Introgressive hybridisation in Heteroptera: the example of Limnoporus Stål (Gerridae) species in western Canada. Can J Zool 68: 1770–1782.
Suzuki TA, Nachman MW . (2015). Speciation and reduced hybrid female fertility in house mice. Evolution 69: 2468–2481.
Tao Y, Hartl DL, Laurie CC . (2001). Sex–ratio segregation distortion associated with reproductive isolation in Drosophila. Proc Natl Acad Sci USA 98: 13183–13188.
Telschow A, Yamamura N, Werren JH . (2005). Bidirectional cytoplasmic incompatibility and the stable coexistence of two Wolbachia strains in parapatric host populations. J Theor Biol 235: 265–274.
Turelli M, Moyle LC . (2007). Asymmetric postmating isolation: Darwin’s corollary to Haldane’s rule. Genetics 176: 1059–1088.
Turelli M, Orr HA . (1995). The dominance theory of Haldane’s rule. Genetics 140: 389–402.
Turelli M, Orr HA . (2000). Dominance, epistasis and the genetics of postzygotic isolation. Genetics 154: 1663–1679.
Venables WN, Ripley BD . (2002) Modern Applied Statistics with S, 4th edn. Springer: New York, NY, USA.
Virdee SR, Hewitt GM . (1992). Postzygotic isolation and Haldane's rule in a grasshopper. Heredity 69: 527–538.
Virdee SR, Hewitt GM . (1994). Clines for hybrid dysfunction in a grasshopper hybrid zone. Evolution 48: 392–407.
Wade MJ, Johnson NA, Toquenaga Y . (1999). Temperature effects and genotype-by-environment interactions in hybrids: Haldane ’ s rule in flour beetles. Evolution 53: 855–865.
Watson ET, Demuth JP . (2012). Haldane’s rule in marsupials: What happens when both sexes are functionally hemizygous? J Hered 103: 453–458.
Woodruff GC, Eke O, Baird SE, Félix MA, Haag ES . (2010). Insights into species divergence and the evolution of hermaphroditism from fertile interspecies hybrids of Caenorhabditis nematodes. Genetics 186: 997–1012.
Wu C, Davis AW . (1993). Evolution of postmating reproductive isolation : the composite nature of Haldane ’ s rule and its genetic bases. Am Naturalist 142: 187–212.
Zeileis A, Kleiber C, Jackman S . (2008). Regression models for Count Data in R. J Stat Software 27: 1–25.
Zuur AF, Saveliev AA, Ieno EN . (2012) Zero Inflated Models snd Generalized Linear Mixed Models with R. Highland Statistics Ltd: Newburgh, NY, USA.
Acknowledgements
We are grateful to the following people for helpful assistance in the field: K Holmes, S Blanksby, M Higgie, G Jones, T Ly, R Ollerynshaw. S Vardy and the Westman family generously provided hospitality and assistance and we appreciate their enthusiasm for our research. We thank four anonymous reviewers and the editor for constructive feedback on the manuscript. D Forbes, A Grant and M McGunnigle helped in the lab. This work was funded by NERC (NE/G014906/1, NE/L011255/1, NE/I027800/1). Additional funding from the Orthopterists’ Society to PM is also gratefully acknowledged.
Data archiving
The doi for our data is doi: 10.5061/dryad.b7k26.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Heredity website
Supplementary information
Rights and permissions
About this article
Cite this article
Moran, P., Ritchie, M. & Bailey, N. A rare exception to Haldane’s rule: Are X chromosomes key to hybrid incompatibilities?. Heredity 118, 554–562 (2017). https://doi.org/10.1038/hdy.2016.127
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hdy.2016.127
This article is cited by
-
Physiological aspects of sex differences and Haldane’s rule in Rumex hastatulus
Scientific Reports (2022)