Abstract
The anti-tumour effects associated with oncolytic virus therapy are mediated significantly through immune-mediated mechanisms, which depend both on the type of virus and the route of delivery. Here, we show that intra-tumoral oncolysis by Reovirus induced the priming of a CD8+, Th1-type anti-tumour response. By contrast, systemically delivered Vesicular Stomatitis Virus expressing a cDNA library of melanoma antigens (VSV-ASMEL) promoted a potent anti-tumour CD4+ Th17 response. Therefore, we hypothesised that combining the Reovirus-induced CD8+ T cell response, with the VSV-ASMEL CD4+ Th17 helper response, would produce enhanced anti-tumour activity. Consistent with this, priming with intra-tumoral Reovirus, followed by an intra-venous VSV-ASMEL Th17 boost, significantly improved survival of mice bearing established subcutaneous B16 melanoma tumours. We also show that combination of either therapy alone with anti-PD-1 immune checkpoint blockade augmented both the Th1 response induced by systemically delivered Reovirus in combination with GM-CSF, and also the Th17 response induced by VSV-ASMEL. Significantly, anti-PD-1 also uncovered an anti-tumour Th1 response following VSV-ASMEL treatment that was not seen in the absence of checkpoint blockade. Finally, the combination of all three treatments (priming with systemically delivered Reovirus, followed by double boosting with systemic VSV-ASMEL and anti-PD-1) significantly enhanced survival, with long-term cures, compared to any individual, or double, combination therapies, associated with strong Th1 and Th17 responses to tumour antigens. Our data show that it is possible to generate fully systemic, highly effective anti-tumour immunovirotherapy by combining oncolytic viruses, along with immune checkpoint blockade, to induce complementary mechanisms of anti-tumour immune responses.
Similar content being viewed by others
Introduction
Oncolytic viruses (OV) are naturally occurring or genetically modified viruses that target tumour cells while largely sparing normal cells, dependent on a number of different mechanisms.1, 2, 3 In this respect, it is now clear that the anti-tumour activity of these agents is, at least in part, dependent on immune responses raised to both the virus and tumour-associated antigens released during the process of immunogenic tumour cell killing.4, 5, 6 This concept is underscored by the recent FDA approval of talimogene laherparepvec (T-Vec, an HSV encoding GM-CSF), confirming the potential of OV as immunovirotherapeutic agents for cancer treatment.
The exact immune mechanisms through which OV induce anti-tumour responses depend upon multiple factors, including the type of virus used, the route of administration of the virus and the transgenes encoded. In this respect, we and others, have shown that immune responses mediated by a range of OV encoding either tumour antigens, cytokines and/or co-stimulatory molecules, are effective in controlling tumour growth in pre-clinical models,7, 8, 9, 10 with several of these agents being tested in clinical trials.11, 12, 13 For example, Reovirus replication occurs in tumour cells with defective anti-viral PKR signalling resulting in oncolysis14 but also generates potent anti-tumour immune responses, both innate and adaptive, which are highly important for tumour regression.15, 16, 17, 18 A number of Phase1/2 clinical trials of Reovirus serotype 3 Dearing (Oncolytics Biotech, Calgary, Canada) have demonstrated it to be safe.19, 20, 21 We have shown that, when delivered intra-tumorally (i.t.), Reovirus generates a Th1 anti-tumour response,22 which also correlates with our previous observations that Reovirus activates cytotoxic T lymphocytes.16, 17 However, when delivered systemically in combination with GM-CSF, we showed that the anti-tumour immune response is also heavily dependent on innate mechanisms.23
We have also developed an effective systemic immunovirotherapy against established tumours using Vesicular Stomatitis Virus (VSV) expressing either single, or multiple, tumour antigens. In particular, intravenous (i.v.) delivery of VSV expressing a cDNA library derived from either normal or tumour cells, primed specific anti-tumour immune responses in models of melanoma, prostate cancer and brain tumours.10, 24, 25 Interestingly, in all of these models, the anti-tumour immune responses primed against tumour by expression of multiple tumour antigens encoded by the virally expressed cDNA were dependent upon CD4+ Th17 cells.10, 24
Normal immune responses to infection or injury are modulated at checkpoints to prevent them leading to uncontrolled immune cell proliferation and auto-immune disease. For example, Programmed cell death-1 (PD-1) is a receptor found on immune cells including T cells, B cells and monocytes26 the binding of which to one of its ligands, PD-L1 or PD-L2, inhibits immune cell activation. Expression of PD-L1 is found on many types of tumour27 resulting in the ability of tumour cells to evade immune responses against them. Checkpoint inhibitors are antibodies that target these negative immune regulators or their ligands, including PD1/PD-L1, and have shown great promise as immune therapy for the treatment of at least a proportion of patients with melanoma and other cancers.28, 29, 30 These data clearly suggest that these checkpoint inhibitors relieve repression of (weak) T cell responses against self-tumour associated antigens, as well as against pathogens associated with infection and injury. Therefore, given that OV can prime anti-tumour T cell responses, several groups have proposed that the combination of OV therapy and checkpoint inhibition will be of immunotherapeutic value.22, 25, 31, 32
In the current study, we hypothesised that a combination of two different forms of oncolytic viroimmunotherapy, which stimulate alternative CD8+ Th1 and CD4+ helper Th17 mechanisms of anti-tumour immunity, could combine cooperatively or synergistically, along with immune checkpoint blockade, to enhance anti-tumour therapy. We show here a Th1/Th17 prime-boost treatment with two different viruses, both delivered systemically, was significantly more effective in controlling tumours than either single immunovirotherapy treatment alone. Further addition of immune checkpoint blockade with anti-PD-1 generated long-term cures in mice treated with the triple combination therapy under experimental conditions where double therapies alone did not.
Results
Reovirus primes a Th1 response, while VSV-cDNA primes a Th17 response against B16 melanoma
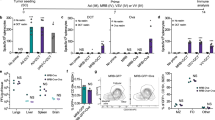
Pooled cultures of splenocytes and lymph node (S/LN) cells from mice treated i.t. with Reovirus, but not with PBS, secreted IFN-γ in response to B16 tumour cell lysates (Figure 1a). They also generated a Th1 recall response to a combination of the three VSV-expressed self-antigens (VSV-NRAS, VSV-CYT-c, VSV-TYRP1), which we have previously described as rejection antigens for B16 tumours following treatment with a VSV-ASMEL cDNA library24 (Figure 1a, VSV-combo). However, no IL-17 (<50 pg/ml, data not shown) was detected as a result of i.t. Reovirus treatment indicating the absence of a Th17 immune response.
Reovirus primes a Th1 response, while VSV-cDNA primes a Th17 response against B16 melanoma. (a, b) C57Bl/6 mice (4 per group) bearing 10-day established B16 tumours, received six i.t. injections of either PBS or Reovirus on days 10, 12, 14, 17, 19, 21 (a), and C57Bl/6 mice (4 per group) bearing 5-day established B16 tumours, received six i.v. injections of either VSV-GFP or VSV-ASMEL on days 5, 7, 9, 12, 14, 16. (b). At day 25, mice were euthanised, spleens and LN dissociated into single cell suspensions and re-stimulated with either: B16 F/T lysate; VSV-NRAS+VSV-CYT-c+VSV-TYRP1 (VSV-combo, total MOI=1 per re-stimulation) or peptide as indicated (1 μg/ml per re-stimulation) every 24 h. Supernatants were harvested after 48 h and tested for IFN-γ and IL-17 by ELISA. Graphs show values +s.d. (triplicate wells) for individual mice. *P<0.05, **P<0.01 two-tailed t-test.
In this subcutaneous (s.c.) B16 model, we have shown that single agent Reovirus delivered i.t., but not i.v., was an effective anti-tumour therapy.33 By contrast, established B16 tumours could be treated with a systemically delivered VSV-cDNA library (VSV-ASMEL – Altered Self-melanoma Eptiope Library).10 The anti-tumour response was dependent on CD4+ T cells and associated with a Th17 response against at least three dominant tumour antigens, NRAS, CYT-c and TYRP1.24 Consistent with those data, splenocyte/LN cells from VSV-ASMEL-treated mice secreted IL-17 in response to either B16 lysate or to the VSV-combo (Figure 1b). By contrast, no IFN-γ was secreted on re-stimulation with B16 lysate or the VSV-combo (<50 pg/ml, data not shown), indicating no significant detectable Th1-type response to this treatment. Therefore, i.t. Reovirus (Th1) and i.v. VSV-cDNA (Th17), prime different types of anti-tumour immune response.
Prime-boost using Reovirus and VSV-ASMEL improves anti-tumour therapy
Therefore, we hypothesized that a combination of immunovirotherapies working through different immune mechanisms would enhance overall anti-tumour therapy in the context of a prime-boost strategy. Using sub-optimal individual treatments either alone, or in combination, to allow detection of improved efficacy, prime-boost with Reo/PBS, Reo/Reo, VSV-ASMEL/VSV-ASMEL, Reo/VSV-GFP and VSV-ASMEL/Reo all resulted in significantly improved survival compared to PBS/PBS-treated controls (Figure 2a, P<0.001 for all). However, prime-boost with Reo/VSV-ASMEL was a significantly better treatment than any of the other regimens (Figure 2a, P<0.001 Reo/VSV-ASMEL vs any other treatment). Increased survival following Reo/VSV-ASMEL prime-boost was associated with a stronger Th1 recall response against B16 lysate, or the melanoma tumour antigen TYRP1, compared to that seen in mice treated with prime-boost Reo/PBS (Figure 2b, P=0.0140, B16 lysate; P=0.0023, TYRP1). There was a trend towards increased Th17 responses following prime-boost Reo/VSV-ASMEL treatment compared to PBS/VSV-ASMEL although this did not reach statistical significance (Figure 2c). IFN-γ or IL-17 recall responses to TC2 F/T lysate, a non-melanoma cell line, were minimal, indicating that the Th1 and Th17 responses were tumour-specific (Figures 2b and c).
Prime-boost using Reovirus and VSV-ASMEL improves anti-tumour therapy. (a) C57Bl/6 mice (7 per group) bearing 10-day established B16 tumours received three i.t. injections of either PBS, Reovirus or VSV-ASMEL on days 10, 12, 14 followed by three i.v. injections of either PBS/Reovirus/VSV-ASMEL on days 17, 19, 21 as indicated. Tumour measurements were taken 3x per week and mice euthanised when tumours reached 1.0 cm diameter. Graph shown is representative of n=2 individual experiments, ***P<0.001 log-rank test Reo/VSV-ASMEL compared to all other groups. (b, c) At time of sacrifice due to tumour burden, S/LN were harvested from three mice per group. Single cell suspension cultures of S/LN were re-stimulated with either B16 (relevant) or TC2 (irrelevant) F/T lysate, or TYRP1 peptide, every 24 h. Supernatants were harvested after 72 h and tested for IFN-γ and IL-17 by ELISA. Bars on graphs show values for individual mice. *P<0.05, **P<0.01 two-tailed t-test.
Enhancement of systemic Reovirus therapy by checkpoint blockade is dependent on CD8+ cells
We have previously shown that systemically delivered Reovirus can be effective when used in combination with other agents such as GM-CSF, cyclophosphamide or VEGF23, 33, 34 or in the context of ex vivo loaded cell carriage.18 In this respect, pre-conditioning with GM-CSF prior to systemic Reovirus delivery effectively treated B16 tumours dependent on innate immune responses.23 As before,23 a suboptimal regimen of two cycles of GM-CSF/Reovirus significantly prolonged survival in C57Bl/6 mice bearing 5-day established B16 s.c. tumours (Figure 3a). Combination with anti-PD-1 checkpoint blockade resulted in significantly improved survival (Figure 3a, GM-CSF/Reovirus/anti-PD-1 vs GM-CSF/Reovirus alone, P=0.0174). We observed a low level Th1 response to tumour antigens following GM-CSF/Reovirus treatment, both by an IFN-γ recall response to B16 tumour lysate (Figure 3b), and by significantly increased numbers of IFN-γ secreting CD8+ T cells (P=0.012) and CD4+ T cells (P=0.009) infiltrating into GM-CSF/Reovirus-treated tumours compared to PBS-treated tumours (Figure 3c). However, this Th1 response was significantly improved by the addition of anti-PD-1 (Figure 3b, GM-CSF/Reovirus/anti-PD-1 vs GM-CSF/Reovirus, P=0.0250). Previously we showed that GM-CSF/Reovirus therapy is largely mediated by innate effectors such as natural killer cells and monocytes.23 Similarly, despite the increased numbers of CD8+ T cells in treated tumours, depletion of neither CD8 nor CD4 cells significantly affected survival after treatment with GM-CSF/Reovirus (Figure 3d). Interestingly, we observed a trend for increased survival with depletion of CD4+ T cells (Figure 3d). Although this was not statistically significant, we are currently testing the hypothesis that depletion of CD4+ T cells leads to removal of a suppressive population, which enhances the innate-immune mediated clearance of tumours induced by GM-CSF/Reovirus treatment. However, consistent with the improved Th1 response seen on addition of anti-PD1 (Figure 3b), depletion of CD8, but not CD4, cells significantly reduced survival in mice treated with GM-CSF/Reovirus+anti-PD-1 (Figure 3e, P=0.0135). No Th17 response was detected following GM-CSF/Reovirus treatment, with, or without, addition of anti-PD-1 (IL-17<20 pg/ml, data not shown). These data suggest that, although the effect of GM-CSF/Reovirus was mainly mediated via innate effectors, a detectable, but low level Th1 response was also generated but did not contribute significantly to tumour control. However, in the presence of checkpoint blockade this weak Th1 response was significantly enhanced, which translated into improved overall survival.
Enhancement of systemic Reovirus therapy by checkpoint blockade is dependent on CD8 cells. (a, b) C57Bl/6 mice (7 per group) bearing 5-day established B16 tumours were treated ±2 cycles of GM-CSF/Reovirus beginning on days 5 and 12, then three injections of anti-PD-1 (250 μg) or control IgG on days 19, 21, 23. (a) Tumours were measured 3x per week and mice euthanised when tumours reached 1.0 cm diameter. *P<0.05 log-rank test. (b) S/LN were harvested at time of sacrifice (as indicated). Single cell suspension cultures of S/LN were re-stimulated with B16 F/T lysate every 24 h. Supernatants were harvested after 72 h and tested for IFN-γ by ELISA. Bars on graphs show values +s.d. (triplicate wells) for individual mice. *P<0.05 two-tailed t-test. (c) Mice with 6-day established s.c. B16 tumours were treated with two rounds of PBS/PBS, PBS/Reo, GM-CSF/PBS or GM-CSF/Reovirus (days 6–10 and 13–17). On day 22 tumours were excised and analysed by intracellular staining for CD3+ CD8+ IFN-γ+, and CD3+ CD4+ IFN-γ+, T cells. The mean percentage of CD3+ CD4+ or CD3+ CD8+ cells, which were also IFN-γ positive, in tumours from each group is shown. Standard deviations represent values from four mice per group (except GM-CSF/PBS where n=3). *P<0.05, **P<0.01 two-tailed t-test. (d, e) C57Bl/6 mice (5 per group) bearing 5-day established B16 tumours, received three cycles of GM-CSF/Reovirus with co-injection of anti-CD4 or anti-CD8 depleting antibodies along with the GM-CSF, beginning on days 5, 12, 19. Anti-PD-1 (250 μg) or control IgG was administered on days 19, 21, 23. Tumours were measured 3x per week and mice euthanised when tumours reached 1.0 cm diameter. (d) Depletion of CD4 or CD8 cells on GM-CSF/Reovirus therapy; (e) Depletion of CD4 or CD8 cells on GM-CSF/Reo/anti-PD-1 therapy. *P<0.05 log-rank test. (d, e) are results from the same experiment.
Checkpoint inhibition improves VSV-ASMEL therapy and uncovers a Th1 anti-tumour response
The addition of anti-PD-1 significantly prolonged survival of mice with established s.c. B16 tumours treated with VSV-ASMEL alone (Figure 4a, VSV-ASMEL+anti-PD-1 vs VSV-ASMEL+control IgG, P=0.018). Improved survival following VSV-ASMEL+anti-PD-1 was associated with a significantly stronger Th17 recall response against B16 lysate compared to VSV-ASMEL alone (Figure 4b, P=0.001). Furthermore, anti-PD-1 treatment uncovered a Th1 response to tumour as evidenced by production of IFN-γ from splenocyte/LN cells in response to B16 lysate (Figure 4c, P=0.0014), which was not detectable in the absence of anti-PD-1.
Checkpoint inhibition improves VSV-ASMEL therapy and uncovers a Th1 anti-tumour response. C57Bl/6 mice (7–8 per group) bearing 5-day established B16 tumours received six injections of either VSV-GFP or VSV-ASMEL on days 5, 7, 9, 12, 14, 16, followed by six injections of anti-PD-1 (250 μg) or control Ig on days 19, 21, 23, 26, 28, 30. (a) Tumour measurements were taken 3x per week and mice euthanised when tumours reached 1.0 cm diameter. Graph shown is representative of n=3 individual experiments, *P<0.05 log-rank test. (b, c) S/LN were harvested from four mice/group at time of sacrifice. Single cell suspension cultures of S/LN were re-stimulated with B16 F/T lysate every 24 h. Supernatants were harvested after 72 h and tested for IL-17 (b) and IFN-γ (c) by ELISA. Bars on graphs show values +s.d. (triplicate wells) for individual mice. **P<0.01, ***P<0.001 two-tailed t-test.
Combined Th1/Th17 therapy, together with checkpoint inhibition, cures B16 melanoma
Finally, we hypothesized that combining an innate-driven/Th1 Reovirus-induced anti-tumour response, with a Th17 VSV-ASMEL-induced response, both of which were enhanced with anti-PD-1 blockade, would generate more effective anti-tumour therapy than either alone. As before, GM-CSF/Reovirus was effective in treating s.c. B16 tumours (Figure 5a, P=0.0004 vs PBS), while combination with anti-PD-1 further improved survival (Figures 3a and 5a). As with i.t. Reovirus+VSV-ASMEL (Figure 2a), prime-boost with systemic GM-CSF/Reovirus followed by VSV-ASMEL was superior to GM-CSF/Reovirus alone (Figure 5a). However, addition of anti-PD-1 to the GM-CSF/Reovirus/VSV-ASMEL prime-boost treatment was the only therapy able to generate long-term cures under these experimental conditions (Figure 5a, P<0.01 vs GM-CSF/Reo, GM-CSF/Reo/anti-PD-1, GM-CSF/VSV-ASMEL). Splenocyte/LN cultures from the long-term cured mice produced significantly higher levels of IFN-γ in response to B16 lysate than mice from any other treatment group, which had been euthanised earlier due to tumour burden (Figure 5b, P=0.00006). This Th1 recall response included a specific component against the melanoma antigen TYRP1 (Figure 5b, P=0.0216 vs control group). In addition, mice treated with GM-CSF/Reovirus/VSV-ASMEL+anti-PD-1 had a significantly improved Th17 recall response compared to those treated with the prime-boost regimen without checkpoint blockade (Figure 5c, P=0.0156). These data show that two separate oncolytic immunovirotherapies working through different immune effector mechanisms, and combined with checkpoint blockade, can be effectively combined to eradicate established disease.
Combined Th1/Th17 therapy, together with checkpoint inhibition, is effective in curing B16 melanoma. C57Bl/6 mice (7 per group) bearing 10-day established B16 tumours received two ‘prime’ cycles of either PBS or GM-CSF/Reovirus starting at days 10 and 17, then three ‘boost’ injections of PBS or VSV-ASMEL on days 24, 26, 28. Anti-PD-1 (225 μg) or control IgG was given on days 24, 26, 28, 31, 33, 35. (a) Tumour measurements were taken 3x per week and mice euthanised when tumours reached 1.0 cm diameter. Graph shown is representative of n=2 individual experiments, **P<0.01 log-rank test. (b, c) S/LN were harvested from three mice/group at time of sacrifice (as indicated in c). Single cell suspension cultures of S/LN were re-stimulated with B16 F/T lysate or peptide as indicated every 24 h. Supernatants were harvested after 72 h and tested for IFN-γ (b) and IL-17 (c) by ELISA. Bars on graphs show values +s.d. (triplicate wells) for individual mice. *P<0.05, ***P<0.001 two-tailed t-test.
Discussion
It is now clear that the efficacy of many OV regimens depends upon an immune component. Thus, Reovirus is effective against B16OVA tumours that are not susceptible to direct oncolysis,17 and systemic VSV did not generate significant anti-tumour therapy in nude mice.35 However, the immunological mechanisms of such effects will vary between virus types, routes of administration and transgenes encoded by the viruses. In this respect, we show here that, whereas i.t. injection of oncolytic Reovirus primed a Th1-type response to B16 s.c. tumours, systemic administration of the VSV-ASMEL cDNA library primed a Th17 response to tumour-specific antigens. Therefore, we hypothesized that combining complementary immunological effector pathways, induced by different OV, would generate improved immune-mediated anti-tumour therapy.
Repeated treatment with the same type of immunovirotherapy (Reo/Reo (Th1) or VSV-ASMEL/VSV-ASMEL (Th17)) resulted in prolonged survival compared to PBS-treated controls (Figure 2a). However, combination Reovirus/VSV-ASMEL (Th1/Th17) prime-boost treatment significantly improved survival compared to repeated single therapies (Figure 2a), associated with enhanced Th1, and, to a lesser extent, Th17 anti-tumour antigen responses, (Figures 2b and c). Interestingly, reversing the order of the prime-boost from Th1/Th17 to Th17/Th1 still significantly improved survival compared to controls. However, this improvement was only comparable to single repeated immunovirotherapies and was significantly less effective than the Th1/Th17 prime-boost (Figure 2a). These data show that two different OV, each priming a different type of immune response, can be combined to produce significantly better therapy than either virus alone. Furthermore, the order in which the responses were induced was important (Th1 followed by Th17).
As part of our long-term goal to develop delivery regimens for oncolytic immunovirotherapy, which do not necessitate direct i.t. injection, we developed an effective systemic Reovirus therapy by pre-conditioning tumour-bearing mice with GM-CSF prior to i.v. Reovirus injection, which is mediated by natural killer cells and CD11b+ monocytes.23 We have also shown that Reovirus-mediated natural killer cell activation following i.t. Reovirus injection was augmented by anti-PD-1 leading to improved tumour therapy.22 Therefore, we investigated whether anti-PD-1 could improve our systemic Reovirus treatment. Figure 3a shows that addition of anti-PD-1 treatment significantly enhanced survival of mice compared to GM-CSF/Reovirus alone. Significantly, this improvement in therapy was associated with an enhanced Th1 response to B16 tumour antigen, which was only minimally detected in the absence of anti-PD-1 (Figure 3b). The improved therapy was also dependent upon CD8+ T cells (Figures 3b and e), consistent with the mechanism of checkpoint blockade as acting predominantly via release of inhibition on T cells.36, 37, 38 These data show that checkpoint blockade mechanistically enhanced systemic GM-CSF/Reovirus therapy by significantly augmenting an otherwise very weak CD8+ T cell-dependent component, which was associated with significantly better anti-tumour therapy.
Similarly, although therapy associated with systemic delivery of VSV-ASMEL was dependent upon CD4+ T cells and a Th17 response (Figure 4b), with no detectable Th1 response (Figure 4c), addition of anti-PD-1 uncovered a Th1 response to tumour antigens that was not detectable in the absence of checkpoint blockade (Figure 4c). As for the addition of anti-PD-1 to the GM-CSF/Reovirus regimen, uncovering of this anti-tumour Th1 response was associated with extended survival, and increased tumour cures, in vivo (Figure 4a). Anti-PD-1 also moderately enhanced the anti-tumour Th17 response against B16 tumour antigen (Figure 4b). We are currently investigating the possibility that anti-PD-1 therapy acts so effectively to augment these otherwise undetectable Th1 T cell responses (for both GM-CSF/Reovirus and VSV-ASMEL treatments), through direct activity on suppressive cells such as MDSC or Treg induced in response to virotherapy.
Since the combination of GM-CSF/Reovirus and VSV-ASMEL therapy enhanced the therapy compared to either alone (Figure 2), and since both mono-immunovirotherapies were significantly enhanced by anti-PD-1 checkpoint inhibition (Figures 3 and 4), we tested the combination of all three therapies. As seen in Figure 5, the triple therapy (GM-CSF/Reovirus (innate immune-mediated, C8+T Th1lo)+VSV-ASMEL boost (CD4+ Th17, Th1lo)+anti-PD-1 (Th1 and Th17 enhancement)) was significantly more effective than any of the double combinations, resulting in tumour regression with 100% of the mice cured long term at day 70, and was associated with very strong Th1 and Th17 responses to tumour antigens, including TYRP-1 (Figure 5). The data of Figures 4b and c and 5b and c show that long-term survival and tumour cure correlated with the development of both anti-tumour Th1 and Th17 recall responses. In contrast, development of either alone, or neither response, was associated with significantly shorter long-term survival. These assays were performed on splenocytes from mice at the time of sacrifice due to tumour burden or at day 100 (Figure 4) or 70 (Figure 5), following tumour seeding for the long-term survivors. We did not perform similar assays on mice at a defined time point following treatment because we believe that the multiple components of the innate and adaptive immune responses that are operative with the full combination therapy would not have developed fully by the early time points at which control-treated mice started to die due to tumour burden.
Our data are consistent with a model in which primary treatment with GM-CSF/Reovirus leads to initial tumour killing through virus delivery and innate immune activation.23 This therapy induced detectable, but very low level, Th1 responses against tumour antigens (Figure 3b). We hypothesise that, critically, initial tumour killing releases a very broad range of tumour antigens, against which only very weak anti-self T cell responses can be primed. Subsequent delivery of VSV-ASMEL provides a similarly broad range of tumour antigens in the form of the cDNA library. These stimulate CD4+ Th17 responses that can, therefore, provide additional help to the T cell responses stimulated by the primary GM-CSF/Reovirus treatment (Figures 2b and c). Finally, late boosting with anti-PD-1 further augments both the already enhanced Th1 and Th17 responses against this broad range of tumour antigens leading to the potent and sustained therapy observed in Figure 5.
Several other groups have also successfully used combinations of OV for tumour therapy consistent with a heterologous prime-boost strategy to generate efficient anti-tumour antigen-specific therapy. For example, rhabdoviruses, such as VSV or Maraba virus, expressing a defined melanoma-associated antigen, provided an effective immunological boost against the antigen in mice previously vaccinated with an adenoviral vector to prime the response.39, 40 Tysome et al showed that sequential treatment with oncolytic adenovirus and vaccinia viruses cured about 60% of tumour bearing Syrian hamsters. Efficacy was dependent upon the sequence of the virus treatments, and, significantly, upon CD3+ T cells, indicating that the combination of viruses was acting through an immunological prime-boost-like mechanism.41 The combination of adenovirus and vaccinia virus was also successful in slowing anti-viral, and innate cellular, immune responses leading to better anti-tumour therapy.42 Similarly, a combination of Semliki Forest Virus and Vaccinia virus was effective at boosting anti-tumour immune responses in a murine ovarian cancer model and generated improved therapy through both oncolysis and enhanced anti-tumour immunity.43 Our approach here moves beyond the use of different vectors encoding specific antigens and uses the release of multiple antigens through oncolysis as the basis of the priming step, which is then boosted by the use of the cDNA library. We believe that raising T cell responses against multiple tumour antigens simultaneously reduces the ability of tumour cells to escape immune pressure by developing antigen loss variants. Our approach here is also novel in that it specifically exploits the complementary immunological mechanisms by which two OV (Reovirus and VSV) stimulate anti-tumour immunity through different immune effectors.
In summary, we show here that it is possible to combine OV, which induce complementary mechanisms of anti-tumour immune responses, along with immune checkpoint blockade, to generate fully systemic, highly effective anti-tumour immunovirotherapy.
Materials and methods
Cell lines
Murine B16 melanoma and TRAMP-C2 (TC2) prostate tumour cells were grown in DMEM (Life Technologies, Carlsbad, CA, USA) supplemented with 10% (v/v) FCS (Life Technologies) and L-glutamine (Life Technologies). Cell lines were monitored routinely and found to be free of Mycoplasma infection.
Viruses
Wild type Reovirus type 3 (Dearing strain, REOLYSIN) was obtained from Oncolytics Biotech. Stock titres were measured by plaque assays on L929 cells. The ASMEL VSV-cDNA library was generated as previously reported.10, 24, 44 Individual viral clones were isolated by limiting dilution as previously described,24, 44 expanded in baby hamster kidney cells and purified by sucrose gradient centrifugation. VSV-GFP was manufactured as described.45
In vivo experiments
Six to eight-week-old female C57Bl/6 mice were purchased from Jackson Laboratories (Bar Harbor, Maine, USA). All in vivo studies were approved by the Mayo IACUC. Mice were challenged subcutaneously with 2 × 105 B16 melanoma cells in 100 μl PBS (HyClone, South Logan, UT, USA). Tumours were measured three times per week, and mice were euthanized when tumours reached 1.0 cm diameter. Reovirus was administered i.v. at 5 × 107 or i.t. at 1 × 108 TCID50 per injection; VSV-GFP and VSV-ASMEL were administered i.v. at 1 × 107 pfu per injection. GM-CSF was administered intra-peritoneally (i.p.) at 300 ng/injection as described previously,23 one cycle of GM-CSF/Reo=GM-CSF i.p. on three consecutive days followed by Reovirus (5 × 107TCID50) i.v. on the following two days. Anti-PD-1 (BioXcell, West Lebanon, NH, USA) or control IgG (BioXcell) was given i.v. at either 225 or 250 μg per injection as detailed in the figure legends. Anti-CD4 (GK1.5, BioXcell) or anti-CD8 antibodies (Lyt2.43, BioXcell) for cell depletions were administered i.p. at 100 μl per injection.
Definitions and dosing regimens used in these studies
VSV-ASMEL
VSV-ASMEL is a VSV-cDNA library expressing cDNA from human melanoma cell lines, which therefore encode altered self-epitopes in the mouse (VSV-ASMEL – Altered Self-melanoma Epitope Library). Previously, we have shown that nine i.v. injections of the VSV-cDNA library (VSV-ASMEL) can cure ~80% of mice with 5-day established, s.c. B16 tumours.24
GM-CSF/Reovirus
This is a treatment schedule in which a single cycle consists of GM-CSF administered i.p. on days 1, 2 and 3 followed by intravenous Reovirus on days 4 and 5. Previously, we have shown that three of these cycles of GM-CSF/Reovirus, over a period of 3 weeks, cured 60–80% of mice with 5-day-established s.c. tumours.23
In the studies reported here, we required models in which either VSV-ASMEL or GM-CSF/Reovirus alone would have diminished therapy, in order to investigate whether combination with each other and/or checkpoint inhibition would enhance therapy. To achieve this, we reduced the starting tumour burden and/or the number of injections of VSV-ASMEL or GM-CSF/Reovirus depending upon the experimental situations as follows:
Tumour burden was increased at the time of treatment from 5-day to 10-day established tumour and the number of systemic injections of VSV-ASMEL was reduced from nine (optimally therapeutic) to three (Figure 2). Under these conditions, i.v. VSV-ASMEL was significantly better than PBS but led to no cures.
Tumour burden was kept at 5-day established s.c. B16 but therapy with GM-CSF/Reovirus was reduced relative to the optimal protocol by administering only two cycles of GM-CSF/Reovirus instead of three (Figure 3). Under these circumstances only ~15% of mice were cured but all mice had significant prolongation of survival before tumours reached 1.0 cm diameter. This condition allowed a significant improvement in therapy with GM-CSF/Reovirus to be shown with the addition of anti-PD-1 therapy.
Tumour burden was kept at 5-day established s.c. B16. However, the number of systemic injections of VSV-ASMEL was reduced from nine (optimally therapeutic) to six (Figure 4). Under these conditions, i.v. VSV-ASMEL was significantly better than i.v. VSV-GFP but led to significantly fewer cures (~25%) than with nine i.v. injections of VSV-ASMEL. This condition allowed a significant improvement in therapy with VSV-ASMEL to be shown with the addition of anti-PD-1 therapy.
Tumour burden was increased at the time of treatment from 5-day to 10-day established tumour (Figure 5). Priming therapy with GM-CSF/Reovirus was reduced relative to the optimal protocol by administering only two cycles of GM-CSF/Reovirus instead of the optimal three. The increased tumour burden made the therapy of GM-CSF/Reovirus+anti-PD-1 less effective here (all mice with tumour by day 70) than the same therapy in Figure 3 (~80% cured of tumour by day 80). The number of systemic injections of VSV-ASMEL was reduced from nine (optimally therapeutic) to just three, which, along with the larger tumour burden, made VSV-ASMEL therapy completely ineffective, which could not be rescued by the addition of anti-PD-1 (VSV-ASMEL/anti-PD-1). This lack of therapy was in contrast to Figure 4 where a smaller starting tumour burden, and more i.v. injections of VSV-ASMEL, generated better single agent therapy, which was enhanced by anti-PD-1. Under these conditions, significant improvement in therapy with either VSV-ASMEL+anti-PD-1 or GM-CSF/Reovirus+anti-PD-1 could be shown with the triple prime-boost combination of GM-CSF/Reovirus+VSV-ASMEL+anti-PD-1.
In vitro splenic re-stimulation of splenocytes/lymph nodes and enzyme-linked immunosorbent assay for IFN-γ/IL-17
Spleen and lymph nodes (S/LN) were immediately excised from euthanized mice and dissociated in vitro to achieve single-cell suspensions. S/LN cells were pooled for each individual mouse. Red blood cells were lysed with ACK lysis buffer for 2 min. Cells were re-suspended in Iscove’s modified Dulbecco’s medium (Gibco, Grand Island, NY, USA)+5% FBS+1% Pen-Strep+40 μM 2-ME. Supernatants were harvested from 1 × 106 S/LN stimulated with one of the following: VSV-combination (VSV-NRAS, VSV-CYT-c, VSV-TYRP1) at MOI=1 per stimulation; 1 μg/ml synthetic H2-b-restricted peptides murine TRP-2180–188 SVYDFFVWL (H2Kb), murine TRP-1222–229 TAYRYHLL (H2Kb), human gp10025–33 (Hgp100) KVPRNQDWL (H2Db), murine gp10025-33 (Mgp100) EGSRNQDWL (H2Db) or with freeze-thaw lysates (equivalent to 1 × 106 tumour cells), from B16 (relevant) or TC2 (irrelevant) tumour cells every 24 h. Cell-free supernatants were collected at 48 or 72 h and tested by enzyme-linked immunosorbent assay for murine IFN-γ or murine IL-17 (BD Biosciences, San Jose, CA, USA). The peptides were synthesized at Mayo Foundation Core Facility (Rochester, MN, USA).
Statistics
Survival data from the animal studies were analysed by the log-rank test using GraphPad Prism 6 Software. A Student’s t-test analysis was applied for in vitro data. Statistical significance was determined at the level of P<0.05.
References
Russell SJ, Peng KW . Measles virus for cancer therapy. Curr Top Microbiol Immunol 2009; 330: 213–241.
Stojdl DF, Lichty BD, tenOever BR, Paterson JM, Power AT, Knowles S et al. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell 2003; 4: 263–275.
Martin TA, Watkins G, Jiang WG . The Coxsackie-adenovirus receptor has elevated expression in human breast cancer. Clin Exp Med 2005; 5: 122–128.
Andtbacka RH, Kaufman HL, Collichio F, Amatruda T, Senzer N, Chesney J et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol 2015; 33: 2780–2788.
Chiocca EA, Rabkin SD . Oncolytic viruses and their application to cancer immunotherapy. Cancer Immunol Res 2014; 2: 295–300.
Kaufman HL, Kohlhapp FJ, Zloza A . Oncolytic viruses: a new class of immunotherapy drugs. Nat Rev Drug Discov 2015; 14: 642–662.
Choi IK, Lee JS, Zhang SN, Park J, Sonn CH, Lee KM et al. Oncolytic adenovirus co-expressing IL-12 and IL-18 improves tumor-specific immunity via differentiation of T cells expressing IL-12Rb2 or IL-18Ra. Gene Ther 2011; 18: 898–909.
Diaconu I, Cerullo V, Hirvinen ML, Escutenaire S, Ugolini M, Pesonen SK et al. Immune response is an important aspect of the antitumor effect produced by a CD40L-encoding oncolytic adenovirus. Cancer Res 2012; 72: 2327–2338.
Kim JH, Oh JY, Park BH, Lee DE, Kim JS, Park HE et al. Systemic armed oncolytic and immunologic therapy for cancer with JX-594, a targeted poxvirus expressing GM-CSF. Mol Ther 2006; 14: 361–370.
Kottke T, Errington F, Pulido J, Galivo F, Thompson J, Wongthida P et al. Broad antigenic coverage induced by vaccination with virus-based cDNA libraries cures established tumors. Nat Med 2011; 17: 854–859.
Pesonen S, Diaconu I, Kangasniemi L, Ranki T, Kanerva A, Pesonen SK et al. Oncolytic immunotherapy of advanced solid tumors with a CD40L-expressing replicating adenovirus: assessment of safety and immunologic responses in patients. Cancer Res 2012; 72: 1621–1631.
Cerullo V, Pesonen S, Diaconu I, Escutenaire S, Arstila PT, Ugolini M et al. Oncolytic adenovirus coding for granulocyte macrophage colony-stimulating factor induces antitumoral immunity in cancer patients. Cancer Res 2010; 70: 4297–4309.
Heo J, Reid T, Ruo L, Breitbach CJ, Rose S, Bloomston M et al. Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nat Med 2013; 19: 329–336.
Coffey MC, Strong JE, Forsyth PA, Lee PW . Reovirus therapy of tumors with activated Ras pathway. Science 1998; 282: 1332–1334.
Errington F, White CL, Twigger KR, Rose A, Scott K, Steele L et al. Inflammatory tumour cell killing by oncolytic reovirus for the treatment of melanoma. Gene Ther 2008; 15: 1257–1270.
Prestwich RJ, Errington F, Ilett EJ, Morgan RS, Scott KJ, Kottke T et al. Tumor infection by oncolytic reovirus primes adaptive antitumor immunity. Clin Cancer Res 2008; 14: 7358–7366.
Prestwich RJ, Ilett EJ, Errington F, Diaz RM, Steele LP, Kottke T et al. Immune-mediated antitumor activity of reovirus is required for therapy and is independent of direct viral oncolysis and replication. Clin Cancer Res 2009; 15: 4374–4381.
Ilett EJ, Prestwich RJ, Kottke T, Errington F, Thompson JM, Harrington KJ et al. Dendritic cells and T cells deliver oncolytic reovirus for tumour killing despite pre-existing anti-viral immunity. Gene Ther 2009; 16: 689–699.
Vidal L, Pandha HS, Yap TA, White CL, Twigger K, Vile RG et al. A phase I study of intravenous oncolytic reovirus type 3 dearing in patients with advanced cancer. Clin Cancer Res 2008; 14: 7127–7137.
Karapanagiotou EM, Roulstone V, Twigger K, Ball M, Tanay M, Nutting C et al. Phase I/II trial of carboplatin and paclitaxel chemotherapy in combination with intravenous oncolytic reovirus in patients with advanced malignancies. Clin Cancer Res 2012; 18: 2080–2089.
Adair RA, Roulstone V, Scott KJ, Morgan R, Nuovo GJ, Fuller M et al. Cell carriage, delivery, and selective replication of an oncolytic virus in tumor in patients. Sci Transl Med 2012; 4: 138ra77.
Rajani K, Parrish C, Kottke T, Thompson J, Zaidi S, Ilett L et al. Combination therapy with reovirus and anti-PD-1 blockade controls tumor growth through innate and adaptive immune responses. Mol Ther 2015; 24: 166–174.
Ilett E, Kottke T, Donnelly O, Thompson J, Willmon C, Diaz R et al. Cytokine conditioning enhances systemic delivery and therapy of an oncolytic virus. Mol Ther 2014; 22: 1851–1863.
Pulido J, Kottke T, Thompson J, Galivo F, Wongthida P, Diaz RM et al. Using virally expressed melanoma cDNA libraries to identify tumor-associated antigens that cure melanoma. Nat Biotechnol 2012; 30: 337–343.
Cockle JV, Rajani K, Zaidi S, Kottke T, Thompson J, Diaz RM et al. Combination viroimmunotherapy with checkpoint inhibition to treat glioma, based on location-specific tumor profiling. Neuro Oncol 2015; 18: 518–527.
Francisco LM, Sage PT, Sharpe AH . The PD-1 pathway in tolerance and autoimmunity. Immunol Rev 2010; 236: 219–242.
Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002; 8: 793–800.
Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015; 373: 23–34.
Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 2014; 515: 558–562.
Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015; 373: 1627–1639.
Zamarin D, Holmgaard RB, Subudhi SK, Park JS, Mansour M, Palese P et al. Localized oncolytic virotherapy overcomes systemic tumor resistance to immune checkpoint blockade immunotherapy. Sci Transl Med 2014; 6: 226ra32.
Engeland CE, Grossardt C, Veinalde R, Bossow S, Lutz D, Kaufmann JK et al. CTLA-4 and PD-L1 checkpoint blockade enhances oncolytic measles virus therapy. Mol Ther 2014; 22: 1949–1959.
Qiao J, Wang H, Kottke T, White C, Twigger K, Diaz RM et al. Cyclophosphamide facilitates antitumor efficacy against subcutaneous tumors following intravenous delivery of reovirus. Clin Cancer Res 2008; 14: 259–269.
Kottke T, Hall G, Pulido J, Diaz RM, Thompson J, Chong H et al. Antiangiogenic cancer therapy combined with oncolytic virotherapy leads to regression of established tumors in mice. J Clin Invest 2010; 120: 1551–1560.
Qiao J, Wang H, Kottke T, Diaz RM, Willmon C, Hudacek A et al. Loading of oncolytic vesicular stomatitis virus onto antigen-specific T cells enhances the efficacy of adoptive T-cell therapy of tumors. Gene Ther 2008; 15: 604–616.
Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012; 366: 2455–2465.
Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014; 515: 568–571.
Karyampudi L, Lamichhane P, Scheid AD, Kalli KR, Shreeder B, Krempski JW et al. Accumulation of memory precursor CD8 T cells in regressing tumors following combination therapy with vaccine and anti-PD-1 antibody. Cancer Res 2014; 74: 2974–2985.
Bridle BW, Clouthier D, Zhang L, Pol J, Chen L, Lichty BD et al. Oncolytic vesicular stomatitis virus quantitatively and qualitatively improves primary CD8 T-cell responses to anticancer vaccines. Oncoimmunol 2013; 2: e26013.
Pol JG, Zhang L, Bridle BW, Stephenson KB, Resseguier J, Hanson S et al. Maraba virus as a potent oncolytic vaccine vector. Mol Ther 2014; 22: 420–429.
Tysome JR, Li X, Wang S, Wang P, Gao D, Du P et al. A novel therapeutic regime to eradicate established solid tumors with an effective induction of tumor-specific immunity. Clin Cancer Res 2012; 18: 6679–6689.
Vaha-Koskela M, Tahtinen S, Gronberg-Vaha-Koskela S, Taipale K, Saha D, Merisalo-Soikkeli M et al. Overcoming tumor resistance by heterologous adeno-poxvirus combination therapy. Mol Ther Oncolytics 2015; 1: 14006.
Zhang YQ, Tsai YC, Monie A, Wu TC, Hung CF . Enhancing the therapeutic effect against ovarian cancer through a combination of viral oncolysis and antigen-specific immunotherapy. Mol Ther 2010; 18: 692–699.
Alonso-Camino V, Rajani K, Kottke T, Rommelfanger-Konkol D, Zaidi S, Thompson J et al. The profile of tumor antigens which can be targeted by immunotherapy depends upon the tumor’s anatomical site. Mol Ther 2014; 22: 1936–1948.
Fernandez M, Porosnicu M, Markovic D, Barber GN . Genetically engineered vesicular stomatitis virus in gene therapy: application for treatment of malignant disease. J Virol 2002; 76: 895–904.
Acknowledgements
We thank Toni Higgins for expert secretarial assistance.
This work was supported by NIH Grants CA175386-01A1 and CA108961-10P3; by the University of Minnesota/Mayo Foundation Partnership Grant; by Oncolytics Biotech (Calgary); The European Research Council Advanced Grant (ONCOVIRAX); Vyriad Pharmaceuticals; and the Mayo Foundation. Richard Vile is in receipt of research funding from Oncolytics.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Ilett, E., Kottke, T., Thompson, J. et al. Prime-boost using separate oncolytic viruses in combination with checkpoint blockade improves anti-tumour therapy. Gene Ther 24, 21–30 (2017). https://doi.org/10.1038/gt.2016.70
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2016.70
This article is cited by
-
Combination therapy with oncolytic viruses and immune checkpoint inhibitors in head and neck squamous cell carcinomas: an approach of complementary advantages
Cancer Cell International (2023)
-
Reovirus combined with a STING agonist enhances anti-tumor immunity in a mouse model of colorectal cancer
Cancer Immunology, Immunotherapy (2023)
-
Oncolytic adenoviruses synergistically enhance anti-PD-L1 and anti-CTLA-4 immunotherapy by modulating the tumour microenvironment in a 4T1 orthotopic mouse model
Cancer Gene Therapy (2022)
-
Delivery of cancer therapies by synthetic and bio-inspired nanovectors
Molecular Cancer (2021)
-
Enhanced anti-melanoma efficacy through a combination of the armed oncolytic adenovirus ZD55-IL-24 and immune checkpoint blockade in B16-bearing immunocompetent mouse model
Cancer Immunology, Immunotherapy (2021)