Abstract
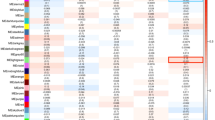
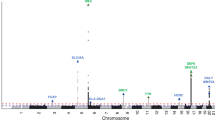
Selective destruction of epidermal melanocytes is central to vitiligo (VL), a common acquired, autoimmune depigmentory disorder of the skin. Like other autoimmune diseases, the pathogenesis of VL is obscure and both multifactorial and polygenic. The prevailing theory is that VL may be part of an autoimmune diathesis. To evaluate mechanisms underlying disease development and progression, we studied genome-wide gene expression from lesional and non-lesional skin of patients with non-segmental VL. Unbiased clustering and principal components analyses reveals a 'lesional pathology'-based signature. Pathway-based analyses of the differentially expressed genes underscore processes such as melanocyte development and cell cycle as central drivers of the disease state. Interactome analysis identifies several key transcriptional regulators potentially affecting disease pathogenesis both within and 'hidden' from the data set. Finally, two genes within six identified transcriptional 'hot spots' coincide with previous VL-associated genetic elements. The remaining genes in the 'hot spots' offer an additional set of potential disease-linked loci that may help to guide future studies aimed at identifying disease risk genes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 digital issues and online access to articles
$119.00 per year
only $19.83 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Boissy RE, Nordlund JJ . Vitiligo: current medical and scientific understanding. G Ital Dermatol Venereol 2011; 146: 69–75.
Nordlund JJ . The epidemiology and genetics of vitiligo. Clin Dermatol 1997; 15: 875–878.
Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ . Fitzpatrick's Dermatol General Medicine, 7th edn, vol. 1. Mac Graw Hill: New York, NY, USA, 2007: pp 616–621.
Hann SK, Park YK, Chun WH . Clinical features of vitiligo. Clin Dermatol 1997; 15: 891–897.
Lerner AB . Vitiligo. J Invest Dermatol 1959; 32: 285–310.
Porter J, Beuf AH, Nordlund JJ, Lerner AB . Psychological reaction to chronic skin disorders: a study of patients with vitiligo. Gen Hosp Psychiatry 1979; 1: 73–77.
Ongenae K, Beelaert L, van Geel N, Naeyaert JM . Psychosocial effects of vitiligo. J Eur Acad Dermatol Venereol 2006; 20: 1–8.
Porter JR, Beuf AH, Lerner AB, Nordlund JJ . The effect of vitiligo on sexual relationships. J Am Acad Dermatol 1990; 22: 221–222.
Silverberg JI, Silverberg NB . Association between vitiligo extent and distribution and quality-of-life impairment. JAMA Dermatol 2013; 149: 159–164.
Schallreuter KU, Bahadoran P, Picardo M, Slominski A, Elassiuty YE, Kemp EH et al. Vitiligo pathogenesis: autoimmune disease, genetic defect, excessive reactive oxygen species, calcium imbalance, or what else? Exp Dermatol 2008; 17: 139–140; discussion 141-60.
Tarle RG, Nascimento LM, Mira MT, Castro CC . Vitiligo—part 1. An Bras Dermatol 2014; 89: 461–470.
Manga P, Kerr R, Ramsay M, Kromberg JG . Biology and genetics of oculocutaneous albinism and vitiligo—common pigmentation disorders in southern Africa. S Afr Med J 2013; 103: 984–988.
Mahdi P, Rouzbahani M, Amali A, Rezaii Khiabanlu S, Kamali M . Audiological manifestations in vitiligo patients. Iran J Otorhinolaryngol 2012; 24: 35–40.
Bulbul Baskan E, Baykara M, Ercan I, Tunali S, Yucel A . Vitiligo and ocular findings: a study on possible associations. J Eur Acad Dermatol Venereol 2006; 20: 829–833.
Rezaei N, Gavalas NG, Weetman AP, Kemp EH . Autoimmunity as an aetiological factor in vitiligo. J Eur Acad Dermatol Venereol 2007; 21: 865–876.
Ongenae K, Van Geel N, Naeyaert JM . Evidence for an autoimmune pathogenesis of vitiligo. Pigment Cell Res 2003; 16: 90–100.
Abdel-Naser MB, Kruger-Krasagakes S, Krasagakis K, Gollnick H, Abdel-Fattah A, Orfanos CE . Further evidence for involvement of both cell mediated and humoral immunity in generalized vitiligo. Pigment Cell Res 1994; 7: 1–8.
Gregg RK, Nichols L, Chen Y, Lu B, Engelhard VH . Mechanisms of spatial and temporal development of autoimmune vitiligo in tyrosinase-specific TCR transgenic mice. J Immunol 2010; 184: 1909–1917.
Harris JE, Harris TH, Weninger W, Wherry EJ, Hunter CA, Turka LA . A mouse model of vitiligo with focused epidermal depigmentation requires IFN-gamma for autoreactive CD8(+) T-cell accumulation in the skin. J Invest Dermatol 2012; 132: 1869–1876.
Oyarbide-Valencia K, van den Boorn JG, Denman CJ, Li M, Carlson JM, Hernandez C et al. Therapeutic implications of autoimmune vitiligo T cells. Autoimmun Rev 2006; 5: 486–492.
Steitz J, Bruck J, Lenz J, Buchs S, Tuting T . Peripheral CD8+ T cell tolerance against melanocytic self-antigens in the skin is regulated in two steps by CD4+ T cells and local inflammation: implications for the pathophysiology of vitiligo. J Invest Dermatol 2005; 124: 144–150.
Le Poole IC, Wankowicz-Kalinska A, van den Wijngaard RM, Nickoloff BJ, Das PK . Autoimmune aspects of depigmentation in vitiligo. J. Investig Dermatol Symp Proc 2004; 9: 68–72.
van den Wijngaard R, Wankowicz-Kalinska A, Le Poole C, Tigges B, Westerhof W, Das P . Local immune response in skin of generalized vitiligo patients. Destruction of melanocytes is associated with the prominent presence of CLA+ T cells at the perilesional site. Lab Invest 2000; 80: 1299–1309.
van den Boorn JG, Konijnenberg D, Dellemijn TA, van der Veen JP, Bos JD, Melief CJ et al. Autoimmune destruction of skin melanocytes by perilesional T cells from vitiligo patients. J Invest Dermatol 2009; 129: 2220–2232.
Harning R, Cui J, Bystryn JC . Relation between the incidence and level of pigment cell antibodies and disease activity in vitiligo. J Invest Dermatol 1991; 97: 1078–1080.
Kemp EH, Waterman EA, Weetman AP . Autoimmune aspects of vitiligo. Autoimmunity 2001; 34: 65–77.
Naughton GK, Eisinger M, Bystryn JC . Antibodies to normal human melanocytes in vitiligo. J Exp Med 1983; 158: 246–251.
Naughton GK, Eisinger M, Bystryn JC . Detection of antibodies to melanocytes in vitiligo by specific immunoprecipitation. J Invest Dermatol 1983; 81: 540–542.
Song YH, Connor E, Li Y, Zorovich B, Balducci P, Maclaren N . The role of tyrosinase in autoimmune vitiligo. Lancet 1994; 344: 1049–1052.
Boniface K, Rezvani HR, Seneschal J, Taieb A . Comment: The mystery of melanocyte demise in vitiligo. Exp Dermatol 2015; 24: 260–261.
Arunachalam M, Colucci R, Berti S, Kline JA, Lotti T, Lotti F et al. Autoimmune signals in non-segmental vitiligo patients are associated with distinct clinical parameters and toxic exposures. J Eur Acad Dermatol Venereol 2013; 27: 961–966.
Mandelcorn-Monson RL, Shear NH, Yau E, Sambhara S, Barber BH, Spaner D et al. Cytotoxic T lymphocyte reactivity to gp100, MelanA/MART-1, and tyrosinase, in HLA-A2-positive vitiligo patients. J Invest Dermatol 2003; 121: 550–556.
Colucci R, Lotti F, Dragoni F, Arunachalam M, Lotti T, Benvenga S et al. High prevalence of circulating autoantibodies against thyroid hormones in vitiligo and correlation with clinical and historical parameters of patients. Br J Dermatol 2014; 171: 786–798.
Yang L, Wei Y, Sun Y, Shi W, Yang J, Zhu L et al. Interferon-gamma inhibits melanogenesis and induces apoptosis in melanocytes: a pivotal role of CD8+ cytotoxic T lymphocytes in vitiligo. Acta Derm Venereol 2015; 95: 664–670.
Rashighi M, Agarwal P, Richmond JM, Harris TH, Dresser K, Su MW et al. CXCL10 is critical for the progression and maintenance of depigmentation in a mouse model of vitiligo. Sci Transl Med 2014; 6: 223ra23.
Tembhre MK, Sharma VK, Sharma A, Chattopadhyay P, Gupta S . T helper and regulatory T cell cytokine profile in active, stable and narrow band ultraviolet B treated generalized vitiligo. Clin Chim Acta 2013; 424: 27–32.
Tembhre MK, Parihar AS, Sharma A, Gupta S, Chattopadhyay P, Sharma VK . Participation of T cell immunoglobulin and mucin domain-3 (TIM-3) and its ligand (galectin-9) in the pathogenesis of active generalized vitiligo. Immunol Res 2015; 62: 23–34.
Khan R, Gupta S, Sharma A . Circulatory levels of T-cell cytokines (interleukin [IL]-2, IL-4, IL-17, and transforming growth factor-beta) in patients with vitiligo. J Am Acad Dermatol 2012; 66: 510–511.
Bassiouny DA, Shaker O . Role of interleukin-17 in the pathogenesis of vitiligo. Clin Exp Dermatol 2011; 36: 292–297.
Nascimento LM, Silva de Castro CC, Fava VM, Iani Werneck R, Mira MT . Genetic and biochemical evidence implicates the butyrylcholinesterase gene BCHE in vitiligo pathogenesis. Exp Dermatol 2015, e-pub ahead of print 17 July 2015; doi:10.1111/exd.12810.
Al-Shobaili HA . Update on the genetics characterization of vitiligo. Int J Health Sci (Qassim) 2011; 5: 167–179.
Ren Y, Yang S, Xu S, Gao M, Huang W, Gao T et al. Genetic variation of promoter sequence modulates XBP1 expression and genetic risk for vitiligo. PLoS Genet 2009; 5: e1000523.
Jin Y, Mailloux CM, Gowan K, Riccardi SL, LaBerge G, Bennett DC et al. NALP1 in vitiligo-associated multiple autoimmune disease. N Engl J Med 2007; 356: 1216–1225.
Dey-Rao R, Smith JR, Chow S, Sinha AA . Differential gene expression analysis in CCLE lesions provides new insights regarding the genetics basis of skin vs systemic disease. Genomics 2014; 104: 144–155.
Dey-Rao R, Sinha AA . Genome-wide transcriptional profiling of chronic cutaneous lupus erythematosus (CCLE) peripheral blood identifies systemic alterations relevant to the skin manifestation. Genomics 2015; 105: 90–100.
Dey-Rao R, Seiffert-Sinha K, Sinha AA . Genome-wide expression analysis suggests unique disease-promoting and disease-preventing signatures in Pemphigus vulgaris. Genes Immun 2013; 14: 487–499.
Yu R, Broady R, Huang Y, Wang Y, Yu J, Gao M et al. Transcriptome analysis reveals markers of aberrantly activated innate immunity in vitiligo lesional and non-lesional skin. PLoS One 2012; 7: e51040.
Ito T, Chiba T, Yoshida M . Exploring the protein interactome using comprehensive two-hybrid projects. Trends Biotechnol 2001; 19: S23–S27.
Wachi S, Yoneda K, Wu R . Interactome-transcriptome analysis reveals the high centrality of genes differentially expressed in lung cancer tissues. Bioinformatics 2005; 21: 4205–4208.
Yang Y, Huang G, Yan X, Qing Z . Clinical analysis of thyroglobulin antibody and thyroid peroxidase antibody and their association with vitiligo. Indian J Dermatol 2014; 59: 357–360.
Afsar FS, Isleten F . Prevalence of thyroid function test abnormalities and thyroid autoantibodies in children with vitiligo. Indian J Endocrinol Metab 2013; 17: 1096–1099.
Khalil A, Zaidman I, Bergman R, Elhasid R, Ben-Arush MW . Autoimmune complications after hematopoietic stem cell transplantation in children with nonmalignant disorders. ScientificWorldJournal 2014; 2014: 581657.
Kim YG, Lee MW, Shin JM, Jeong MG, Ko JY . Colocalization of nonsegmental vitiligo and extragenital lichen sclerosus in a 45-year-old female patient with Hashimoto's thyroiditis. J Dermatol 2015; 42: 333–4.
Zhong Y, Kinio A, Saleh M . Functions of NOD-like receptors in human diseases. Front Immunol 2013; 4: 333.
Orlow SJ . Melanosomes are specialized members of the lysosomal lineage of organelles. J Invest Dermatol 1995; 105: 3–7.
Delevoye C, Hurbain I, Tenza D, Sibarita JB, Uzan-Gafsou S, Ohno H et al. AP-1 and KIF13A coordinate endosomal sorting and positioning during melanosome biogenesis. J Cell Biol 2009; 187: 247–264.
Ingordo V, Cazzaniga S, Raone B, Digiuseppe MD, Musumeci ML, Fai D et al. Circulating autoantibodies and autoimmune comorbidities in vitiligo patients: a multicenter Italian study. Dermatology 2014; 228: 240–249.
Colucci R, Dragoni F, Moretti S . Oxidative stress and immune system in vitiligo and thyroid diseases. Oxid Med Cell Longev 2015; 2015: 7 63192.
Diaz-Angulo S, Lopez-Hoyos M, Munoz-Cacho P, Lopez-Escobar M, Gonzalez-Lopez MA . High prevalence of thyroid autoimmunity in patients with alopecia areata and vitiligo: a controlled study. Australas J Dermatol 2015; 56: 142–143.
Zhou H, Zhao J, Tang X, Zhang X, He D . Autoimmune hyperthyroidism, vitiligo, halo nevus and lupus. Am J Med Sci 2015, e-pub ahead of print 4 July 2015; doi:10.1097/MAJ.0000000000000526.
Huang Y, Yi X, Jian Z, Wei C, Li S, Cai C et al. A single-nucleotide polymorphism of miR-196a-2 and vitiligo: an association study and functional analysis in a Han Chinese population. Pigment Cell Melanoma Res 2013; 26: 338–347.
Picardo M, Taieb A Vitiligo. Springer-Verlag: Heidelberg, 2010.
Kroll TM, Bommiasamy H, Boissy RE, Hernandez C, Nickoloff BJ, Mestril R et al. 4-Tertiary butyl phenol exposure sensitizes human melanocytes to dendritic cell-mediated killing: relevance to vitiligo. J Invest Dermatol 2005; 124: 798–806.
Shroads AL, Coats BS, McDonough CW, Langaee T, Stacpoole PW . Haplotype variations in glutathione transferase zeta 1 influence the kinetics and dynamics of chronic dichloroacetate in children. J Clin Pharmacol 2015; 55: 50–55.
Wang S, Liu D, Ning W, Xu A . Cytosolic dsDNA triggers apoptosis and pro-inflammatory cytokine production in normal human melanocytes. Exp Dermatol 2015; 24: 298–300.
Lee AY . Role of keratinocytes in the development of vitiligo. Ann Dermatol 2012; 24: 115–125.
Yu N, Zhang S, Sun T, Kang K, Guan M, Xiang L . Double-stranded RNA induces melanocyte death via activation of Toll-like receptor 3. Exp Dermatol 2011; 20: 134–139.
Coda AB, Sinha AA . Integration of genome-wide transcriptional and genetic profiles provides insights into disease development and clinical heterogeneity in alopecia areata. Genomics 2011; 98: 431–439.
Manga P, Sheyn D, Yang F, Sarangarajan R, Boissy RE . A role for tyrosinase-related protein 1 in 4-tert-butylphenol-induced toxicity in melanocytes: implications for vitiligo. Am J Pathol 2006; 169: 1652–1662.
Jin Y, Birlea SA, Fain PR, Ferrara TM, Ben S, Riccardi SL et al. Genome-wide association analyses identify 13 new susceptibility loci for generalized vitiligo. Nat Genet 2012; 44: 676–680.
Chen JP, Li HP, Jin SH, Zhang JT, Li J . [Detection of serum autoantibodies to melanocyte and correlation between melanoma antigen recognized by T-cells and vitiligo in children]. Nan Fang Yi Ke Da Xue Xue Bao 2009; 29: 2107–2108, 2111.
Silva de Castro CC, do Nascimento LM, Walker G, Werneck RI, Nogoceke E, Mira MT . Genetic variants of the DDR1 gene are associated with vitiligo in two independent Brazilian population samples. J Invest Dermatol 2010; 130: 1813–1818.
Schallreuter KU, Elwary SM, Gibbons NC, Rokos H, Wood JM . Activation/deactivation of acetylcholinesterase by H2O2: more evidence for oxidative stress in vitiligo. Biochem Biophys Res Commun 2004; 315: 502–508.
Tu CX, Gu JS, Lin XR . Increased interleukin-6 and granulocyte-macrophage colony stimulating factor levels in the sera of patients with non-segmental vitiligo. J Dermatol Sci 2003; 31: 73–78.
Spencer JD, Gibbons NC, Bohm M, Schallreuter KU . The Ca2+-binding capacity of epidermal furin is disrupted by H2O2-mediated oxidation in vitiligo. Endocrinology 2008; 149: 1638–1645.
Na GY, Lee KH, Kim MK, Lee SJ, Kim DW, Kim JC . Polymorphisms in the melanocortin-1 receptor (MC1R) and agouti signaling protein (ASIP) genes in Korean vitiligo patients. Pigment Cell Res 2003; 16: 383–387.
Gottumukkala RV, Waterman EA, Herd LM, Gawkrodger DJ, Watson PF, Weetman AP et al. Autoantibodies in vitiligo patients recognize multiple domains of the melanin-concentrating hormone receptor. J Invest Dermatol 2003; 121: 765–770.
Spencer JD, Gibbons NC, Rokos H, Peters EM, Wood JM, Schallreuter KU . Oxidative stress via hydrogen peroxide affects proopiomelanocortin peptides directly in the epidermis of patients with vitiligo. J Invest Dermatol 2007; 127: 411–420.
Xia Q, Zhou WM, Liang YH, Ge HS, Liu HS, Wang JY et al. MHC haplotypic association in Chinese Han patients with vitiligo. J Eur Acad Dermatol Venereol 2006; 20: 941–946.
Casp CB, She JX, McCormack WT . Genes of the LMP/TAP cluster are associated with the human autoimmune disease vitiligo. Genes Immun 2003; 4: 492–499.
Li M, Sun D, Li C, Zhang Z, Gao L, Li K et al. Functional polymorphisms of the FAS gene associated with risk of vitiligo in Chinese populations: a case-control analysis. J Invest Dermatol 2008; 128: 2820–2824.
Aydingoz IE, Kanmaz-Ozer M, Gedikbasi A, Vural P, Dogru-Abbasoglu S, Uysal M . The combination of tumour necrosis factor-alpha -308 A and interleukin-10 -1082G gene polymorphisms and increased serum levels of related cytokines: susceptibility to vitiligo. Clin Exp Dermatol 2015; 40: 71–77.
Abanmi A, Al Harthi F, Zouman A, Kudwah A, Jamal MA, Arfin M et al. Association of interleukin-10 gene promoter polymorphisms in Saudi patients with vitiligo. Dis Markers 2008; 24: 51–57.
Abanmi A, Al Harthi F, Al Baqami R, Al Assaf S, Zouman A, Arfin M et al. Association of HLA loci alleles and antigens in Saudi patients with vitiligo. Arch Dermatol Res 2006; 298: 347–352.
Tursen U, Kaya TI, Erdal ME, Derici E, Gunduz O, Ikizoglu G . Association between catechol-O-methyltransferase polymorphism and vitiligo. Arch Dermatol Res 2002; 294: 143–146.
Schallreuter KU, Rubsam K, Gibbons NC, Maitland DJ, Chavan B, Zothner C et al. Methionine sulfoxide reductases A and B are deactivated by hydrogen peroxide (H2O2) in the epidermis of patients with vitiligo. J Invest Dermatol 2008; 128: 808–815.
Spritz RA, Gowan K, Bennett DC, Fain PR . Novel vitiligo susceptibility loci on chromosomes 7 (AIS2) and 8 (AIS3), confirmation of SLEV1 on chromosome 17, and their roles in an autoimmune diathesis. Am J Hum Genet 2004; 74: 188–191.
Dwivedi M, Laddha NC, Shajil EM, Shah BJ, Begum R . The ACE gene I/D polymorphism is not associated with generalized vitiligo susceptibility in Gujarat population. Pigment Cell Melanoma Res 2008; 21: 407–408.
Song GG, Bae SC, Kim JH, Lee YH . The angiotensin-converting enzyme insertion/deletion polymorphism and susceptibility to rheumatoid arthritis, vitiligo and psoriasis: a meta-analysis. J Renin-Angiotensin-Aldosterone Syst 2013; 16: 195–202.
Namian AM, Shahbaz S, Salmanpoor R, Namazi MR, Dehghani F, Kamali-Sarvestani E . Association of interferon-gamma and tumor necrosis factor alpha polymorphisms with susceptibility to vitiligo in Iranian patients. Arch Dermatol Res 2009; 301: 21–25.
Em S, Laddha NC, Chatterjee S, Gani AR, Malek RA, Shah BJ et al. Association of catalase T/C exon 9 and glutathione peroxidase codon 200 polymorphisms in relation to their activities and oxidative stress with vitiligo susceptibility in Gujarat population. Pigment Cell Res 2007; 20: 405–407.
Yasar A, Gunduz K, Onur E, Calkan M . Serum homocysteine, vitamin B12, folic acid levels and methylenetetrahydrofolate reductase (MTHFR) gene polymorphism in vitiligo. Dis Markers 2012; 33: 85–89.
Uhm YK, Yoon SH, Kang IJ, Chung JH, Yim SV, Lee MH . Association of glutathione S-transferase gene polymorphisms (GSTM1 and GSTT1) of vitiligo in Korean population. Life Sci 2007; 81: 223–227.
Blomhoff A, Kemp EH, Gawkrodger DJ, Weetman AP, Husebye ES, Akselsen HE et al. CTLA4 polymorphisms are associated with vitiligo, in patients with concomitant autoimmune diseases. Pigment Cell Res 2005; 18: 55–58.
Zhu Y, Wang S, Lin F, Li Q, Xu A . The therapeutic effects of EGCG on vitiligo. Fitoterapia 2014; 99C: 243–251.
Mosenson JA, Eby JM, Hernandez C, Le Poole IC . A central role for inducible heat-shock protein 70 in autoimmune vitiligo. Exp Dermatol 2013; 22: 566–569.
Dwivedi M, Laddha NC, Mansuri MS, Marfatia YS, Begum R . Association of NLRP1 genetic variants and mRNA overexpression with generalized vitiligo and disease activity in a Gujarat population. Br J Dermatol 2013; 169: 1114–1125.
Spritz RA . The genetics of generalized vitiligo and associated autoimmune diseases. Pigment Cell Res 2007; 20: 271–278.
Spritz RA . The genetics of generalized vitiligo. Curr Dir Autoimmun 2008; 10: 244–257.
Spritz RA . Modern vitiligo genetics sheds new light on an ancient disease. J Dermatol 2013; 40: 310–318.
Kingo K, Aunin E, Karelson M, Philips MA, Ratsep R, Silm H et al. Gene expression analysis of melanocortin system in vitiligo. J Dermatol Sci 2007; 48: 113–122.
Li S, Yao W, Pan Q, Tang X, Zhao S, Wang W et al. Association analysis revealed one susceptibility locus for vitiligo with immune-related diseases in the Chinese Han population. Immunogenetics 2015; 67: 347–354.
Tang XF, Zhang Z, Hu DY, Xu AE, Zhou HS, Sun LD et al. Association analyses identify three susceptibility Loci for vitiligo in the Chinese Han population. J Invest Dermatol 2013; 133: 403–410.
Rahner N, Hoefler G, Hogenauer C, Lackner C, Steinke V, Sengteller M et al. Compound heterozygosity for two MSH6 mutations in a patient with early onset colorectal cancer, vitiligo and systemic lupus erythematosus. Am J Med Genet A 2008; 146A: 1314–1319.
Nair BK . Vitiligo—a retrospect. Int J Dermatol 1978; 17: 755–757.
Dwivedi M, Helen Kemp E, Laddha NC, Mansuri MS, Weetman AP, Begum R . Regulatory T cells in vitiligo: implications for pathogenesis and therapeutics. Autoimmun Rev 2015; 14: 49–56.
Arend WP, Palmer G, Gabay C . IL-1, IL-18, and IL-33 families of cytokines. Immunol Rev 2008; 223: 20–38.
Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 2005; 23: 479–490.
Moulin D, Donze O, Talabot-Ayer D, Mezin F, Palmer G, Gabay C . Interleukin (IL)-33 induces the release of pro-inflammatory mediators by mast cells. Cytokine 2007; 40: 216–225.
Li P, Ma H, Han D, Mou K . Interleukin-33 affects cytokine production by keratinocytes in vitiligo. Clin Exp Dermatol 2015; 40: 163–170.
Moretti S, Spallanzani A, Amato L, Hautmann G, Gallerani I, Fabbri P . Vitiligo and epidermal microenvironment: possible involvement of keratinocyte-derived cytokines. Arch Dermatol 2002; 138: 273–274.
Levandowski CB, Mailloux CM, Ferrara TM, Gowan K, Ben S, Jin Y et al. NLRP1 haplotypes associated with vitiligo and autoimmunity increase interleukin-1beta processing via the NLRP1 inflammasome. Proc Natl Acad Sci USA 2013; 110: 2952–2956.
Pedra JH, Cassel SL, Sutterwala FS . Sensing pathogens and danger signals by the inflammasome. Curr Opin Immunol 2009; 21: 10–16.
Schroder K, Tschopp J . The inflammasomes. Cell 2010; 140: 821–832.
Yang CA, Chiang BL . Inflammasomes and human autoimmunity: a comprehensive review. J Autoimmun 2015; 61: 1–8.
Nold-Petry CA, Lo CY, Rudloff I, Elgass KD, Li S, Gantier MP et al. IL-37 requires the receptors IL-18Ralpha and IL-1R8 (SIGIRR) to carry out its multifaceted anti-inflammatory program upon innate signal transduction. Nat Immunol 2015; 16: 354–365.
Tu CX, Jin WW, Lin M, Wang ZH, Man MQ . Levels of TGF-beta(1) in serum and culture supernatants of CD4(+)CD25 (+) T cells from patients with non-segmental vitiligo. Arch Dermatol Res 2011; 303: 685–689.
Zhou L, Shi YL, Li K, Hamzavi I, Gao TW, Huggins RH et al. Increased circulating Th17 cells and elevated serum levels of TGF-beta and IL-21 are correlated with human non-segmental vitiligo development. Pigment Cell Melanoma Res 2015; 28: 324–329.
Wang CQ, Cruz-Inigo AE, Fuentes-Duculan J, Moussai D, Gulati N, Sullivan-Whalen M et al. Th17 cells and activated dendritic cells are increased in vitiligo lesions. PLoS One 2011; 6: e18907.
Ando H, Niki Y, Ito M, Akiyama K, Matsui MS, Yarosh DB et al. Melanosomes are transferred from melanocytes to keratinocytes through the processes of packaging, release, uptake, and dispersion. J Invest Dermatol 2012; 132: 1222–1229.
Stenmark H . Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol 2009; 10: 513–525.
Chung H, Jung H, Lee JH, Oh HY, Kim OB, Han IO et al. Keratinocyte-derived laminin-332 protein promotes melanin synthesis via regulation of tyrosine uptake. J Biol Chem 2014; 289: 21751–21759.
Bertolotto C, Abbe P, Hemesath TJ, Bille K, Fisher DE, Ortonne JP et al. Microphthalmia gene product as a signal transducer in cAMP-induced differentiation of melanocytes. J Cell Biol 1998; 142: 827–835.
Otreba M, Rok J, Buszman E, Wrzesniok D . [Regulation of melanogenesis: the role of cAMP and MITF]. Postepy Hig Med Dosw (Online) 2012; 66: 33–40.
Kingo K, Aunin E, Karelson M, Ratsep R, Silm H, Vasar E et al. Expressional changes in the intracellular melanogenesis pathways and their possible role in the pathogenesis of vitiligo. J Dermatol Sci 2008; 52: 39–46.
Slominski A, Tobin DJ, Shibahara S, Wortsman J . Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev 2004; 84: 1155–1228.
Cui J, Shen LY, Wang GC . Role of hair follicles in the repigmentation of vitiligo. J Invest Dermatol 1991; 97: 410–416.
Yamada T, Hasegawa S, Inoue Y, Date Y, Yamamoto N, Mizutani H et al. Wnt/beta-catenin and kit signaling sequentially regulate melanocyte stem cell differentiation in UVB-induced epidermal pigmentation. J Invest Dermatol 2013; 133: 2753–2762.
Regazzetti C, Joly F, Marty C, Rivier M, Mehul B, Reiniche P et al. Transcriptional analysis of vitiligo skins reveals the alteration of WNT pathway: a promising target for repigmenting vitiligo patients. J Invest Dermatol 2015, e-pub ahead of print 31 August 2015; doi:10.1038/jid.2015.335.
Coda AB, Qafalijaj Hysa V, Seiffert-Sinha K, Sinha AA . Peripheral blood gene expression in alopecia areata reveals molecular pathways distinguishing heritability, disease and severity. Genes Immun 2010; 11 (7): 531–541.
Walker A, Mesinkovska NA, Boncher J, Tamburro J, Bergfeld WF . Colocalization of vitiligo and alopecia areata presenting as poliosis. J Cutan Pathol 2015; 42: 150–154.
Mohan GC, Silverberg JI . Association of vitiligo and alopecia areata with atopic dermatitis: a systematic review and meta-analysis. JAMA Dermatol 2015; 151: 522–528.
Chen YT, Chen YJ, Hwang CY, Lin MW, Chen TJ, Chen CC et al. Comorbidity profiles in association with vitiligo: a nationwide population-based study in Taiwan. J Eur Acad Dermatol Venereol 2014; 29: 1362–1369.
Larue L, Delmas V . The WNT/Beta-catenin pathway in melanoma. Front Biosci 2006; 11: 733–742.
Bellei B, Pitisci A, Catricala C, Larue L, Picardo M . Wnt/beta-catenin signaling is stimulated by alpha-melanocyte-stimulating hormone in melanoma and melanocyte cells: implication in cell differentiation. Pigment Cell Melanoma Res 2011; 24 (2): 309–325.
Levy C, Khaled M, Fisher DE . MITF: master regulator of melanocyte development and melanoma oncogene. Trends Mol Med 2006; 12: 406–414.
Eichhoff OM, Weeraratna A, Zipser MC, Denat L, Widmer DS, Xu M et al. Differential LEF1 and TCF4 expression is involved in melanoma cell phenotype switching. Pigment Cell Melanoma Res 2011; 24: 631–642.
Gauthier Y, Cario Andre M, Taieb A . A critical appraisal of vitiligo etiologic theories. Is melanocyte loss a melanocytorrhagy? Pigment Cell Res 2003; 16: 322–332.
Huang CL, Nordlund JJ, Boissy R . Vitiligo: a manifestation of apoptosis? Am J Clin Dermatol 2002; 3: 301–308.
Grandori C, Cowley SM, James LP, Eisenman RN . The Myc/Max/Mad network and the transcriptional control of cell behavior. Annu Rev Cell Dev Biol 2000; 16: 653–699.
Aravalli RN, Talbot NC, Steer CJ . Gene expression profiling of MYC-driven tumor signatures in porcine liver stem cells by transcriptome sequencing. World J Gastroenterol 2015; 21: 2011–2029.
Ramana CV, Grammatikakis N, Chernov M, Nguyen H, Goh KC, Williams BR et al. Regulation of c-myc expression by IFN-gamma through Stat1-dependent and -independent pathways. EMBO J 2000; 19: 263–272.
Ott G . Impact of MYC on malignant behavior. Hematology Am Soc Hematol Educ Program 2014; 2014: 100–106.
Liu GY, Luo Q, Xiong B, Pan C, Yin P, Liao HF et al. Tissue array for Tp53, C-myc, CCND1 gene over-expression in different tumors. World J Gastroenterol 2008; 14: 7199–7207.
Utikal J, Leiter U, Udart M, Kaskel P, Peter RU, Krahn GM . Expression of c-myc and bcl-2 in primary and advanced cutaneous melanoma. Cancer Invest 2002; 20: 914–921.
Shishodia S, Harikumar KB, Dass S, Ramawat KG, Aggarwal BB . The guggul for chronic diseases: ancient medicine, modern targets. Anticancer Res 2008; 28: 3647–3664.
Dang CV . MYC on the path to cancer. Cell 2012; 149: 22–35.
Swindell WR, Sarkar MK, Stuart PE, Voorhees JJ, Elder JT, Johnston A et al. Psoriasis drug development and GWAS interpretation through in silico analysis of transcription factor binding sites. Clin Transl Med 2015; 4: 13.
Loganzo F Jr, Dosik JS, Zhao Y, Vidal MJ, Nanus DM, Sudol M et al. Elevated expression of protein tyrosine kinase c-Yes, but not c-Src, in human malignant melanoma. Oncogene 1993; 8: 2637–2644.
Brautigan DL . Flicking the switches: phosphorylation of serine/threonine protein phosphatases. Semin Cancer Biol 1995; 6: 211–217.
Deichmann M, Polychronidis M, Wacker J, Thome M, Naher H . The protein phosphatase 2 A subunit Bgamma gene is identified to be differentially expressed in malignant melanomas by subtractive suppression hybridization. Melanoma Res 2001; 11: 577–585.
Petritsch C, Beug H, Balmain A, Oft M . TGF-beta inhibits p70 S6 kinase via protein phosphatase 2 A to induce G(1) arrest. Genes Dev 2000; 14: 3093–3101.
Tobin DJ, Swanson NN, Pittelkow MR, Peters EM, Schallreuter KU . Melanocytes are not absent in lesional skin of long duration vitiligo. J Pathol 2000; 191: 407–416.
Hirobe T . Role of keratinocyte-derived factors involved in regulating the proliferation and differentiation of mammalian epidermal melanocytes. Pigment Cell Res 2005; 18: 2–12.
Sviderskaya EV, Wakeling WF, Bennett DC . A cloned, immortal line of murine melanoblasts inducible to differentiate to melanocytes. Development 1995; 121: 1547–1557.
Richmond JM, Frisoli ML, Harris JE . Innate immune mechanisms in vitiligo: danger from within. Curr Opin Immunol 2013; 25: 676–682.
Chen R, Morgan AA, Dudley J, Deshpande T, Li L, Kodama K et al. FitSNPs: highly differentially expressed genes are more likely to have variants associated with disease. Genome Biol 2008; 9: R170.
Waterman EA, Kemp EH, Gawkrodger DJ, Watson PF, Weetman AP . Autoantibodies in vitiligo patients are not directed to the melanocyte differentiation antigen MelanA/MART1. Clin Exp Immunol 2002; 129: 527–532.
Jin Y, Hayashi M, Fain PR, Suzuki T, Fukai K, Oiso N et al. Major association of vitiligo with HLA-A*02:01 in Japanese. Pigment Cell Melanoma Res 2015; 28: 360–362.
Agarwal P, Rashighi M, Essien KI, Richmond JM, Randall L, Pazoki-Toroudi H et al. Simvastatin prevents and reverses depigmentation in a mouse model of vitiligo. J Invest Dermatol 2015; 135: 1080–1088.
Wu Z, Irizarry RA . Preprocessing of oligonucleotide array data. Nat Biotechnol 2004; 22: 656–658; author reply 658.
Huang, da W, Sherman BT, Lempicki RA . Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 2009; 4: 44–57.
Huang,, Sherman BT, Tan Q, Kir J, Liu D, Bryant D et al. DAVID Bioinformatics Resources: expanded annotation database and novel algorithms to better extract biology from large gene lists. Nucleic Acids Res 2007; 35: W169–W175.
Rivals I, Personnaz L, Taing L, Potier MC . Enrichment or depletion of a GO category within a class of genes: which test? Bioinformatics 2007; 23: 401–407.
Shmelkov E, Tang Z, Aifantis I, Statnikov A . Assessing quality and completeness of human transcriptional regulatory pathways on a genome-wide scale. Biol Direct 2011; 6: 15.
Bessarabova M, Ishkin A, JeBailey L, Nikolskaya T, Nikolsky Y . Knowledge-based analysis of proteomics data. BMC Bioinformatics 2012; 13: S13.
Ideker T, Ozier O, Schwikowski B, Siegel AF . Discovering regulatory and signalling circuits in molecular interaction networks. Bioinformatics 2002; 18: S233–S240.
Acknowledgements
We thank BK Sinha and M Sinha for continued guidance and support. This work was supported in part through grants to AAS from the National Vitiligo Foundation and the American Vitiligo Research Foundation.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Genes and Immunity website
Supplementary information
Rights and permissions
About this article
Cite this article
Dey-Rao, R., Sinha, A. Interactome analysis of gene expression profile reveals potential novel key transcriptional regulators of skin pathology in vitiligo. Genes Immun 17, 30–45 (2016). https://doi.org/10.1038/gene.2015.48
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gene.2015.48
This article is cited by
-
Construction of a Comprehensive Protein–Protein Interaction Map for Vitiligo Disease to Identify Key Regulatory Elements: A Systemic Approach
Interdisciplinary Sciences: Computational Life Sciences (2018)
-
Vitiligo blood transcriptomics provides new insights into disease mechanisms and identifies potential novel therapeutic targets
BMC Genomics (2017)