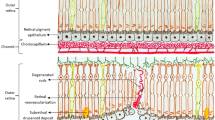
Abstract
This review summarises our current understanding of the molecular basis of subretinal neovascularisation (SRNV) in age-related macular degeneration (AMD). The term neovascular AMD (NVAMD) is derived from the dominant early clinical features of haemorrhage, fluid, and lipid in the subretinal space (SRS) and the historical role of fluorescein angiography in detecting the presence of NV tissue. However, at the cellular level, SRNV resembles an aberrant but stereotypical tissue repair response that incorporates both an early inflammatory phase and a late fibrotic phase in addition to the neovascular (NV) component that dominates the early clinical presentation. This review will seek not only to highlight the important molecules involved in each of these components but to demonstrate that the development of SRNV has its origins in the earliest events in non-NV AMD pathogenesis. Current evidence suggests that this early-stage pathogenesis is characterised by complement-mediated immune dysregulation, leading to a state of chronic inflammation in the retinal pigment epithelium/Bruch’s membrane/choriocapillaris complex. These initial events can be seamlessly and inextricably linked to late-stage development of SRNV in AMD by the process of dynamic reciprocity (DyR), the ongoing bidirectional communication between cells, and their surrounding matrix. Moreover, this correlation between disease onset and eventual outcome is reflected in the temporal and spatial correlation between chronic inflammation, NV, and fibrosis within the reparative microenvironment of the SRS. In summary, the downstream consequences of the earliest dysfunctional molecular events in AMD can result in the late-stage entity we recognize clinically as SRNV and is characterized by a spectrum of predictable, related, and stereotypical processes referred to as DyR.
Similar content being viewed by others
Introduction
Age-related macular degeneration (AMD) remains one of the biggest ongoing vision-related public health issues in the developed world.1 With the ageing population, the burden on society from AMD will likely continue to rise not only in terms of treatment but also in funding ongoing research and in the provision of services for those rendered blind by the condition.2 AMD, which most commonly affects those over the age of 60 years, is a continuum of chronic inflammatory disease. In advanced cases, it is associated with degeneration of the photoreceptors resulting from either progressive degeneration of the retinal pigment epithelium (RPE) and Bruch’s membrane (BM), termed geographic atrophy, or the development of subretinal neovascularisation (SRNV) and termed neovascular AMD (NVAMD).3 Drusen are the earliest clinical hallmark of the disease and are extracellular deposits that build up between the RPE and BM.4 They consist of aggregations of intracellular, extracellular, and secreted proteins together with lipids and other cellular components such as double-stranded RNAs.4, 5, 6, 7 Current evidence implicates dysfunctionality of inflammatory activation pathways, in particular the complement pathway, as being pivotal in contributing to the development of AMD.8, 9, 10, 11 SRNV most commonly takes the form of choroidal neovascularisation (CNV), the ingrowth of NV tissue through BM into the subretinal space (SRS), or occasionally retinal angiomatous proliferation, the ingrowth of NV tissue from either the deep capillary plexus of the retina or the choroid also into the SRS but which has a predilection for the neurosensory retina (NSR).12 Currently, the only effective therapy is for NVAMD and although this affects modest numbers (10–15% of advanced forms of AMD9, 10, 11, 13, 14, 15) compared with non-NVAMD (NNVAMD), it carries a disproportionate risk for patients in terms of severity of vision loss.16, 17, 18, 19, 20 Anti-angiogenic therapy (also called anti-vascular endothelial growth factor (VEGF) therapy) is now the gold standard for the treatment of NVAMD.16, 17, 18, 19 Over the past number of years, it has revolutionized our ability to not only preserve but also improve vision in many sufferers. Meanwhile, several clinical trials are evaluating newer agents as treatments for NNVAMD but for this larger group of patients, dietary supplements as per the Age-Related Eye Disease Study data remain their only recourse to what is essentially a low-efficacy intervention.21, 22, 23
Microglia, resident inflammatory cells of the retina, contributes to retinal homestasis by migrating from their normal perivascular location in the inner layers of the NSR to the SRS where they assist in the normal elimination of visual byproducts.24 With increasing age, these cells assume an activated morphology possibly as a response to chronic oxidative stress associated with ageing that originates within the RPE. Activated microglial cells produce various cytokines that lead to the recruitment of monocytes. In addition, there is an age-related impairment in microglial migration, leading to their accumulation within the SRS.24 The overall result is chronic immune activation. It is possibly at this juncture that the alternative complement pathway assumes its pathological role in the development of AMD. Single-nucleotide polymorphisms in complement factor H and other components of the alternative pathway lead to less regulation of the pathway and the promotion of a hyperinflammatory state, the downstream consequences of which is the eventual development of AMD.25, 26, 27, 28, 29
This mini review summarises our current understanding of the molecular basis underlying NVAMD and how the earliest pathological events in AMD are inextricably linked to the late-stage entity we recognise as NVAMD by a process called dynamic reciprocity (DyR). At the cellular level, NVAMD is in fact an aberrant tissue repair response, characterized not only by NV but also by inflammation and fibrosis as evidenced by the identification of molecular markers other than those involved in NV formation and progression.30, 31, 32, 33, 34, 35 The fact that we refer to a condition characterized by aberrant repair as NVAMD reflects the clinical and angiographic dominance of NV in this disease process.36, 37 If terminology is important, then subretinal repair (SRR) is probably a more accurate pathological description than SRNV to describe this late stage of AMD.38 Furthermore, when describing the formation of new blood vessels in SRR/SRNV, the term neovascularisation is preferred to angiogenesis, because neovascularisation encompasses the contribution of bone marrow-derived cells in the repair process.34, 38 However, for the purposes of this study the terms NVAMD and SRNV will be used interchangeably.
Inflammation
From a wound-healing perspective, the precise sequence of molecular events leading to the development of SRNV in AMD is uncertain, because conventional wound-healing models are based mainly on what occurs in the skin.35 Yet, the processes in the skin and the submacular space bear a striking resemblance to each other, therefore making it reasonable to extrapolate occurrences in the skin to the submacular space.30, 31, 32, 33 The fact that there are similarities between the two locations is not too surprising, because tissue repair is such a stereotypical process regardless of the tissues involved.32 Unlike the skin, the submacular space in not routinely accessible to biopsy material. However, surgical excision of SRNV demonstrates the presence of a provisional matrix of both fibrin and fibronectin, major components of the early wound-healing matrix that are not seen in the later maturational stage of SRNV development.31, 39, 40, 41, 42 Furthermore, other wound-healing matricellular proteins such as secreted protein acidic and rich in cysteine, tenacin, and thrombospondin are present in CNV and again are key factors in early matrix formation, and are involved in a number of key RPE and monocyte functions such as adhesion, proliferation, and migration.43, 44, 45, 46, 47, 48 Certainly, the presence of the RPE cells with their elaboration of a multitude of cytokines and growth factors, including transforming growth factor-β (TGF-β), fibroblast growth factor (FGF)-1, FGF-2, and tumour necrosis factor-α (TNF-α), provide many of the essential ingredients to prime the whole reparative process towards completion.32 In the skin, these same mediators are present in abundance in both the inflammatory and granulation phase of wound healing, again highlighting the stereotypical wound-healing process occurring in SRNV.49, 50, 51, 52, 53 Meanwhile, it is the elaboration of TNF-α by newly arrived monocytes/macrophages that promotes the release of interleukin (IL)-8 and monocyte chemoattractant protein (MCP)-1 by RPE, with the MCP-1 probably amplifying macrophage infiltration.30, 54, 55, 56 TNF-α expression by these CNV-associated macrophages also stimulates the production of VEGF by RPE, as well as placental growth factor (PIGF), platelet-derived growth factor (PDGF)-β, and stromal-derived factor (SDF)-1.52, 57, 58, 59, 60, 61, 62 VEGF heralds the onset of the proliferative phase of tissue repair of which two cells are pivotal, endothelial cells and de-differentiated RPE, the former of course involved in NV and the latter in recruitment of disciform scar tissue.
Subretinal neovascularisation
The foregoing outlines the role of inflammation in both the first stage of wound healing and the necessary process to create the milieu conducive to NV (proliferative phase). The first pillar of wound healing dictates that to activate a reparative response an ischaemic or hypoxic insult is necessary.35 Despite this, there is as yet no direct evidence of either retinal or choroidal hypoxia/ischaemia underlying the development of SRNV in AMD.38, 63 Yet, it is not inconceivable that there are localized micropockets of hypoxia in what is essentially the diseased microenvironment of the submacula and this will trigger the initial inflammation, as outlined in the previous section, that is required to promote an ECM conducive to supporting NV.64, 65, 66, 67 Alternatively, stabilization of hypoxic-inducible factor (HIF)-1 and, therefore, the promotion of NV may be triggered by the AMD-associated pathological oxidative stress within the RPE and photoreceptors, and this in turn will provoke the necessary initial inflammation that primes repair and leads to NV.23, 68, 69 Moreover, activation of HIF transcription factors are being increasingly implicated in inflammation, repair, fibrosis, and epithelial-to-mesenchymal transition, all pivotal features of chronic inflammatory diseases, including AMD.70 Whatever the precise mechanism, hypoxic or non-hypoxic, the induction of the key transcription factor HIF-1 upregulates the expression of hypoxia-regulated genes and their products, including PIGF, PDGF-β, and SDF-1 in addition to VEGF.58, 59, 60, 61, 62 Of course the expression of SDF-1 and VEGF also highlights the role of vasculogenesis in SRNV, although again the precise mechanism of how this is achieved is unclear.34, 63, 71
The role of VEGF in NV is also intimately associated with the angiopoietins and their tyrosine kinase receptors Tie-1 and Tie-2 that are expressed on endothelial cells and also on some macrophage subtypes involved in angiogenesis.72 Initially, Ang-2 expression, in concert with VEGF, promotes vascular instability and promotion of endothelial cell sprouting. As neovascularisation develops, blood vessel formation proceeds from immature endothelial-lined tubes to a mature vascular plexus. In normal tissue repair, basement membrane deposition and recruitment of pericytes heralds the onset of both vascular competence and onset of vessel maturation. Ang-1 and its receptor Tie-2 are fundamental to this maturation process by promoting adhesion and survival of endothelial cells. The completion of the wound-healing process sees levels of VEGF decline, and in the absence of VEGF and Ang-2, probably in conjunction with Ang-1, there is promotion of vascular regression, maturation, and stability.73, 74
Scarring
Scars are the result of a complete healing response. However, clearly with AMD we can argue that the formation of scar tissue (disciform) is an undesirable endpoint, because it appears excessive with the exuberant fibrosis leading to irreversible loss of photoreceptors.75 Even though PDGF is produced early in repair by platelets and injured capillaries, there is ample evidence of its major role in scar tissue formation and fibrosis. In cutaneous healing, PDGF, via its receptors and activation of tyrosine kinase, is a potent mitogen of fibroblasts, stimulates the production of the ECM, and also induces the myofibroblast phenotype.76 In the SRS, PDGF could be the pivotal factor in promoting the RPE fibroblast phenotype that similarly divides and contributes to pathological matrix formation.77 Other evidence suggests that aberrant scar tissue formation may also be related to early recruitment of CD4+ T-helper cells. This T-helper response can be either predominantly T-helper 1 (TH1) or T-helper 2 (TH2). The TH2 response via the elaboration of IL-4, IL-5, IL-10, and IL-13 has been linked to fibrogenesis, whereas the possible beneficial effects on fibrosis by TH1 cells may result from the promotion of pro-apoptotic genes, collagenase activity, and matrix remodelling.78, 79, 80, 81 As fibrosis is such a dominant component of NVAMD, it suggests that the immune system may have a role in AMD, and that TH2 cells may be the predominant T-helper cell involved.82, 83, 84 In contrast to TH1 cells, TH2-linked genes include procollagen I, III, and V, matrix metalloproteinase-2 and 9 (MMP2 and MMP9), and tissue inhibitors of MMP-1 (TIMP-1).85
Similar to T-helper cells, there appears to be at least two distinct types of macrophages, M1 and M2, involved in repair and both are deployed within the first 24 h in the inflammatory phase of wound healing.86, 87, 88, 89 The first type, activated by TH1 cytokines, degrade matrix and produce pro-inflammatory cytokines. The second type, activated by TH2 cytokines and apoptotic cells, produce anti-inflammatory cytokines such as IL-4 as well as TGF-β, and participates in immunoregulation in addition to repair.90, 91 In a murine model of liver fibrosis, for example, the amount of fibrosis seems to be dependent on whether macrophage depletion is early (less fibrosis) or late (more fibrosis), suggesting that functionally distinct sub-populations exist in the same fibrotic tissue.88 This raises the possibility that targeting early CNV in AMD may be important in terms of therapeutically modifying late-onset scar tissue recruitment.92 Moreover, it raises several other questions. First, could one of the mechanisms by which current anti-VEGF therapy prevent scar tissue recruitment be indirectly through its effects on macrophages?93, 94 Second, would it be more beneficial to influence monocyte/macrophage behaviour even earlier in the evolution of AMD, before the onset of NV? Finally, do the conflicting results of previous studies of the role of macrophages in various models of NVAMD accurately mimic the disease in humans?95, 96, 97, 98
TGF-β is a multi-functional cytokine and is usually secreted in its latent form, thus allowing for sustained release throughout the repair process.76 It can both stimulate and inhibit mitogenesis and stimulate angiogenesis, fibroblast differentiation, and matrix deposition.99 Early in acute injury, TGF-β1 and 2 are released by platelets and act as potent chemoattractants for neutrophils, macrophages, and fibroblasts. These cells in turn secrete further TGF-β, thus amplifying its overall effect. The association of TGF-β1 and 2 with excessive scar tissue formation is well established because of elevated and persistent levels of the cytokine in fibroblasts associated with hypertrophic scars and keloid formation.100 In fetal scarless wounds, TGF is only transiently expressed, whereas fetal wounds treated with TGF generates scar tissue formation.101 TGF-β3 on the other hand is expressed in the later stages of healing and reduces ECM deposition.101 Furthermore, the anti-fibrotic role of decorin in fibrogenesis is being increasingly recognised.102, 103, 104, 105, 106, 107, 108, 109 Decorin is a small proteoglycan that modulates collagen, fibronectin, and glycosaminoglycan synthesis by binding to and neutralizing TGF-β.110 The fact that low levels of decorin are found in hypertrophic scar tissue suggests that an imbalance between it and TGF-β produces a more pro-fibrotic state.111, 112
Timely breakdown and remodelling of the ECM is also an important component of normal repair and is regulated by MMPs, a family of structurally related enzymes. Secreted in an inactive form, they are transcriptionally regulated by cytokines, growth factors, cell–cell, and cell–matrix interactions.113 MMP activities are further regulated by the activation of precursor zymogens and curtailed by their endogenous inhibitor TIMPs.114 Ultimately, it is the balance between MMPs and TIMPs that appear crucial for remodelling of the ECM. Yet, another process by which tissue repair in the submacular space could modulate scar tissue formation is through the process of collagen-contraction/remodelling-induced apoptosis (CrIA). For example, fibroblasts seeded on an in-vitro collagen matrix undergo CrIA following contraction and remodelling of the gel.115, 116 Therefore, it is not unreasonable to extrapolate that de-differentiated fibroblastic RPE, which behave similar to fibroblasts in in-vitro models of repair, will do likewise in AMD.117 In general, as a wound matures and deposition of scar tissue occurs, there is a gradual decrease in fibroblast cell density that eventually resembles normal non-injured tissue cellularity.88 This process is also mediated in part by the induction of apoptosis, whereas in aberrant scarring, such as NVAMD, inadequate apoptosis may occur.118, 119, 120
Similar to that in the earlier stages of repair, the bone marrow is also intrinsically involved in fibrogenesis. Fibrocytes in the peripheral blood are derived from mesenchymal stem cells in the bone marrow and are involved not only in scar tissue deposition but are also thought to have a role in the inflammatory and proliferative (NV) phases of repair.121 Similar to fibroblasts in morphology, their haematopoietic origin is confirmed by the expression of CD11b, CD13, CD34, and CD45 markers.122, 123 They have a multifunctional role that includes the secretion of cytokines, growth factors and chemokines, the promotion of angiogenesis, the synthesis of collagen and fibronectin, and are involved in scar tissue contraction and remodelling through MMP production.122, 124 In pathological repair, bone marrow-derived peripheral blood mononuclear cells more readily differentiate to fibrocytes whereas the fibrotic tissue itself contains more of these cells than the normal scar tissue.125 Finally, fibrocytes can act as precursors of fibroblasts, which can also be involved in aberrant scarring.122
Dynamic reciprocity
The foregoing provides a general description of the molecular events involved in NVAMD in the context of a wound-healing response. It can be seen that the entire process we refer to clinically as SRNV involves much more that NV. Initially, there is the chronic inflammation that defines NNVAMD that can eventually lead to SRNV, which without treatment will give rise to fibrosis and irreversible loss of vision. In short, AMD involves a seamless transition between early asymptomatic disease that can lead to an end-stage reparative process (NVAMD), which without therapeutic intervention will almost always have destructive consequences for the retina and vision. At the molecular level, the initiation, coordination, progression, and termination of this entire process can be understood and explained by the concept of DyR. At its most, fundamental repair involves interactions between cells and the immediate ECM. It is through these interactive processes that cell behaviours, such as differentiation/de-differentiation, proliferation, and migration are modified. This ongoing, continuously evolving, bidirectional ‘cross-talk’ between cells and the ECM is referred to as DyR.126 It is with this concept of DyR that we can interpret and understand how the key components of inflammation, neovascularisation, and scarring are seamlessly interlinked and continuously evolving in the wound microenvironment that characterizes NVAMD (Figure 1).127, 128, 129, 130, 131, 132, 133 Without an initial injury, there is no inflammation and therefore no proliferative phase of healing, and consequently no NV, no scar tissue formation, and ultimately no wound quiescence. Integrins are the key cell membrane components that facilitate this bidirectional communication between a cell and its matrix. Integrins are transmembrane multi-domain receptors whose extracellular portion interacts with ECM molecules, while the intracellular portion interacts with protein signalling pathways linked to gene expression in the nucleus and cytoskeletal adjustments on the cell surface.134, 135 If normal wound healing involves an orderly transition arising from cell–matrix interactions via a multitude of different signalling pathways, is it an alteration in the sequence, in the directionality, or timing of these pathways that gives rise to aberrant repair such as we see in NVAMD?
DyR: the algorithm is similar between acute and chronic injury, but with completely different clinical outcomes. With an acute injury to the RPE (eg, therapeutic thermal macular laser), there is restoration of normal or near-normal retinal architecture and function. Chronic injury (eg, AMD) induces chronic inflammation and therefore a pathological outcome. This maladaptation occurs early in the process with predictable consequences namely pathological neovascularisation and fibrosis in the case of NVAMD. Untreated, this stereotypical cascade of molecular events is manifested clinically as the equally stereotypical disciform scar. Abbreviations: AMD, age-related macular degeneration; NV, neovascular; RPE, retinal pigment epithelium.
Bringing it all together: AMD and DyR
Fundamentally, AMD is a chronic inflammatory disease that eventually leads to SRNV in ~10% of cases.9, 10, 11, 13, 14, 15 In general, the basic tenets of inciting a wound-healing response are present.32 However, by definition, chronic wounds are wounds that do not heal and in the context of DyR the cross-talk between cells and matrix is dysfunctional once chronic inflammation becomes established in the early asymptomatic stage of NNVAMD. It is a testament to the durability of repair in these hostile conditions that patients can maintain normal or near-normal vision despite potentially decades of chronic inflammatory dysfunction that is recognized clinically by the presence of drusen, atrophy, and pigmentation within the retina. This in turn suggests that future therapies should be ideally delivered early in the disease, perhaps even years before symptoms arise. In short, the normal reparative sequence is hindered virtually from the outset due to absence of the normal matrix and cells within the RPE/BM/choriocapillaris (CC) complex and this in turn gives rise to further undesirable downstream cell–matrix interactions that delay or prevent healing. In other words, the process of DyR can have either a physiological (restoration of normal or near-normal tissue) or a pathological outcome (disciform scar formation and irreversible destruction of the photoreceptors) that is dependent on the health or otherwise of the tissue within which the wound-healing response is activated (Figure 1). In AMD, the neovessels (SRNV) associated with the proliferative phase of repair are not only abnormal with respect to their location but are also characterized by decreased capillary density, altered morphology, and increased permeability, and is such a microangiopathy rather than a normal NV response occurring as part of physiologic wound healing.136 Moreover, bleeding from these vessels, which is not a feature of normal repair, will compound the dysfunctional reparative response in an increasingly disordered matricellular environment that ultimately leads to aggressive end-stage fibrosis.75 The injury in NVAMD is therefore a ‘two-hit’ process. The first ‘hit’ occurs secondary to the primary underlying pathology that causes the chronic inflammatory process, which gives rise to AMD in the first place, whereas the second ‘hit’ results from recurrent bleeding from SRNV (microangiopathy) and the additional inflammatory process generated from the presence of this extravascular blood. It is interesting to speculate whether it is the presence of this blood that ultimately generates the exuberant fibrosis that we recognise clinically as disciform scarring.75
Based on the concept of DyR, we can therefore postulate the following sequence of events in AMD (Figure 2). The RPE/BM/CC complex is the homeostatic support system for the outer NSR. During everyday maintenance, each RPE cell is exposed to oxidative stress. This stress is maximal in the macular area and is physiological in the sense that each cell employs efficient ‘housekeeping’ mechanisms to maintain normal cellular activity.24 However, with ageing and/or in those who are genetically predisposed, and/or those exposed to environmental insults such as smoking, this physiological oxidative stress can become pathological and overwhelm normal cellular coping mechanisms, leading to build up of RPE waste products.69 Alternatively, age-related hypoxia may induce pathological oxidative stress.68 Either way, we can speculate that intracellular hypoxia occurs and this generates a wound-healing response possibly within the RPE. Initially, this will incite acute inflammation; however, as the injurious agent (pathological oxidative stress/chronic hypoxia) persists, inflammation becomes chronic. Although originating within the confines of the cell, the process of DyR dictates that it will spill out into the ECM and so a pathological feedback loop between cell and matrix, which could include an immune component, is established (bidirectional cross-talk), giving rise to a pathological molecular cascade ending in GA or NVAMD, or both.82, 83, 84, 136 The abnormal local response may also compromise the systemic input from the bone marrow or both local and systemic components could be blunted by co-existent disease.34, 71, 121, 137
DyR and AMD: the exposure of genetically predisposed individuals to environmental risk factors converts physiological oxidative stress to the pathological equivalent, triggering a molecular cascade that ultimately leads to end-stage AMD as defined by the process of DyR. Abbreviation: AMD, age-related macular degeneration.
In summary, it appears that the process that is DyR generates the stereotypical and even predictable wound response that is seen in AMD. As outlined in the previous sections of this study, all the necessary molecular ingredients appear to be present, to generate a normal reparative response. However, as DyR is occurring in a chronically inflamed and disordered RPE/BM/CC environment, it ensures that the repair process in AMD is dysfunctional almost from the outset. Moreover, although SRNV dominates the clinical picture, inflammation and fibrogenesis are equally important in overall disease progression and therefore future research and therapeutics must also be directed at these key components. DyR is the stereotypical seamless molecular transition that links all three components that should allow the prediction, both spatially and temporally, of the key molecules, which are expressed at the various stages in the progression of AMD and could in turn permit the construction of more appropriate therapeutic algorithms that can be customised according to the specific molecular staging of AMD.
References
Bressler NM . Age-related macular degeneration is the leading cause of blindness. JAMA 2004; 291 (15): 1900–1901.
Wong WL, Su X, Li X, Cheung CM, Klein R, Cheng CY et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health 2014; 2 (2): e106–e116.
Ambati J, Ambati BK, Yoo SH, Ianchulev S, Adamis AP . Age-related macular degeneration: etiology, pathogenesis, and therapeutic strategies. Surv Ophthalmol 2003; 48 (3): 257–293.
Curcio CA, Messinger JD, Sloan KR, McGwin G, Medeiros NE, Spaide RF . Subretinal drusenoid deposits in non-neovascular age-related macular degeneration: morphology, prevalence, topography, and biogenesis model. Retina 2013; 33 (2): 265–276.
Crabb JW, Miyagi M, Gu X, Shadrach K, West KA, Sakaguchi H et al. Drusen proteome analysis: an approach to the etiology of age-related macular degeneration. Proc Natl Acad Sci USA 2002; 99 (23): 14682–14687.
Johnson LV, Leitner WP, Rivest AJ, Staples MK, Radeke MJ, Anderson DH . The Alzheimer’s A beta -peptide is deposited at sites of complement activation in pathologic deposits associated with aging and age-related macular degeneration. Proc Natl Acad Sci USA 2002; 99 (18): 11830–11835.
Kaneko H, Dridi S, Tarallo V, Gelfand BD, Fowler BJ, Cho WG et al. DICER1 deficit induces Alu RNA toxicity in age-related macular degeneration. Nature 2011; 471 (7338): 325–330.
Ambati J, Atkinson JP, Gelfand BD . Immunology of age-related macular degeneration. Nat Rev Immunol 2013; 13 (6): 438–451.
Hageman GS, Anderson DH, Johnson LV, Hancox LS, Taiber AJ, Hardisty LI et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci USA 2005; 102 (20): 7227–7232.
Haines JL, Hauser MA, Schmidt S, Scott WK, Olson LM, Gallins P et al. Complement factor H variant increases the risk of age-related macular degeneration. Science 2005; 308 (5720): 419–421.
Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C et al. Complement factor H polymorphism in age-related macular degeneration. Science 2005; 308 (5720): 385–389.
Freund KB, Ho IV, Barbazetto IA, Koizumi H, Laud K, Ferrara D et al. Type 3 neovascularization: the expanded spectrum of retinal angiomatous proliferation. Retina 2008; 28 (2): 201–211.
Edwards AO, Ritter R 3rd, Abel KJ, Manning A, Panhuysen C, Farrer LA . Complement factor H polymorphism and age-related macular degeneration. Science 2005; 308 (5720): 421–424.
Forrester JV . Bowman lecture on the role of inflammation in degenerative disease of the eye. Eye (Lond) 2013; 27 (3): 340–352.
Ferris FL 3rd, Fine SL, Hyman L . Age-related macular degeneration and blindness due to neovascular maculopathy. Arch Ophthalmol 1984; 102 (11): 1640–1642.
Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP, Ianchulev T . Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: Two-year results of the ANCHOR study. Ophthalmology 2009; 116 (1): 57–65.
Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, Jaffe GJ . Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med 2011; 364 (20): 1897–1908.
Regillo CD, Brown DM, Abraham P, Yue H, Ianchulev T, Schneider S et al. Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER Study year 1. Am J Ophthalmol 2008; 145 (2): 239–248.
Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006; 355 (14): 1419–1431.
Argon laser photocoagulation for senile macular degeneration. Results of a randomized clinical trial. Arch Ophthalmol 1982; 100 (6): 912–918.
Age-Related Eye Disease Study 2 Research Group. Lutein+zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA 2013; 309 (19): 2005–2015.
Yehoshua Z, Alexandre de Amorim Garcia Filho C, Nunes RP, Gregori G, Penha FM, Moshfeghi AA et al. Systemic complement inhibition with eculizumab for geographic atrophy in age-related macular degeneration: the COMPLETE Study. Ophthalmology 2014; 121 (3): 693–701.
Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol 2001; 119 (10): 1417–1436.
Xu H, Chen M, Forrester JV . Para-inflammation in the aging retina. Prog Retin Eye Res 2009; 28 (5): 348–368.
Clark SJ, Perveen R, Hakobyan S, Morgan BP, Sim RB, Bishop PN et al. Impaired binding of the age-related macular degeneration-associated complement factor H 402H allotype to Bruch's membrane in human retina. J Biol Chem 2010; 285 (39): 30192–30202.
Johnson PT, Betts KE, Radeke MJ, Hageman GS, Anderson DH, Johnson LV . Individuals homozygous for the age-related macular degeneration risk-conferring variant of complement factor H have elevated levels of CRP in the choroid. Proc Natl Acad Sci USA 2006; 103 (46): 17456–17461.
Laine M, Jarva H, Seitsonen S, Haapasalo K, Lehtinen MJ, Lindeman N et al. Y402H polymorphism of complement factor H affects binding affinity to C-reactive protein. J Immunol 2007; 178 (6): 3831–3836.
Sjoberg AP, Trouw LA, Clark SJ, Sjolander J, Heinegard D, Sim RB et al. The factor H variant associated with age-related macular degeneration (His-384) and the non-disease-associated form bind differentially to C-reactive protein, fibromodulin, DNA, and necrotic cells. J Biol Chem 2007; 282 (15): 10894–10900.
Tuo J, Grob S, Zhang K, Chan CC . Genetics of immunological and inflammatory components in age-related macular degeneration. Ocul Immunol Inflamm 2012; 20 (1): 27–36.
Grossniklaus HE, Ling JX, Wallace TM, Dithmar S, Lawson DH, Cohen C et al. Macrophage and retinal pigment epithelium expression of angiogenic cytokines in choroidal neovascularization. Mol Vis 2002; 8: 119–126.
Grossniklaus HE, Martinez JA, Brown VB, Lambert HM, Sternberg Jr P, Capone Jr A et al. Immunohistochemical and histochemical properties of surgically excised subretinal neovascular membranes in age-related macular degeneration. Am J Ophthalmol 1992; 114 (4): 464–472.
Kent D, Sheridan C . Choroidal neovascularization: a wound healing perspective. Mol Vis 2003; 9: 747–755.
Sheridan CM, Pate S, Hiscott P, Wong D, Pattwell DM, Kent D . Expression of hypoxia-inducible factor-1alpha and -2alpha in human choroidal neovascular membranes. Graefes Arch Clin Exp Ophthalmol 2009; 247 (10): 1361–1367.
Sheridan CM, Rice D, Hiscott PS, Wong D, Kent DL . The presence of AC133-positive cells suggests a possible role of endothelial progenitor cells in the formation of choroidal neovascularization. Invest Ophthalmol Vis Sci 2006; 47 (4): 1642–1645.
Singer AJ, Clark RA . Cutaneous wound healing. N Engl J Med 1999; 341 (10): 738–746.
Subfoveal neovascular lesions in age-related macular degeneration. Guidelines for evaluation and treatment in the macular photocoagulation study. Macular Photocoagulation Study Group (see comments). Arch Ophthalmol 1991; 109 (9): 1242–1257.
Chamberlin JA, Bressler NM, Bressler SB, Elman MJ, Murphy RP, Flood TP et al. The use of fundus photographs and fluorescein angiograms in the identification and treatment of choroidal neovascularization in the Macular Photocoagulation Study. The Macular Photocoagulation Study Group. Ophthalmology 1989; 96 (10): 1526–1534.
Kent DL . Age-related macular degeneration: beyond anti-angiogenesis. Mol Vis 2014; 20: 46–55.
Grossniklaus HE, Hutchinson AK, Capone Jr A, Woolfson J, Lambert HM . Clinicopathologic features of surgically excised choroidal neovascular membranes. Ophthalmology 1994; 101 (6): 1099–1111.
Grossniklaus HE, Green WR . Histopathologic and ultrastructural findings of surgically excised choroidal neovascularization. Submacular Surgery Trials Research Group. Arch Ophthalmol 1998; 116 (6): 745–749.
Lopez PF, Grossniklaus HE, Lambert HM, Aaberg TM, Capone Jr A, Sternberg Jr P et al. Pathologic features of surgically excised subretinal neovascular membranes in age-related macular degeneration. Am J Ophthalmol 1991; 112 (6): 647–656.
Saxe SJ, Grossniklaus HE, Lopez PF, Lambert HM, Sternberg Jr P, L'Hernault N . Ultrastructural features of surgically excised subretinal neovascular membranes in the ocular histoplasmosis syndrome. Arch Ophthalmol 1993; 111 (1): 88–95.
Nicolo M, Piccolino FC, Zardi L, Giovannini A, Mariotti C . Detection of tenascin-C in surgically excised choroidal neovascular membranes. Graefes Arch Clin Exp Ophthalmol 2000; 238 (2): 107–111.
Miyajima-Uchida H, Hayashi H, Beppu R, Kuroki M, Fukami M, Arakawa F et al. Production and accumulation of thrombospondin-1 in human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 2000; 41 (2): 561–567.
Mousa SA, Lorelli W, Campochiaro PA . Role of hypoxia and extracellular matrix-integrin binding in the modulation of angiogenic growth factors secretion by retinal pigmented epithelial cells. J Cell Biochem 1999; 74 (1): 135–143.
Greenwood JA, Murphy-Ullrich JE . Signaling of de-adhesion in cellular regulation and motility. Microsc Res Tech 1998; 43 (5): 420–432.
Bornstein P . Diversity of function is inherent in matricellular proteins: an appraisal of thrombospondin 1. J Cell Biol 1995; 130 (3): 503–506.
Gailit J, Clark RA . Wound repair in the context of extracellular matrix. Curr Opin Cell Biol 1994; 6 (5): 717–725.
Amin R, Puklin JE, Frank RN . Growth factor localization in choroidal neovascular membranes of age-related macular degeneration. Invest Ophthalmol Vis Sci 1994; 35 (8): 3178–3188.
Lopez PF, Sippy BD, Lambert HM, Thach AB, Hinton DR . Transdifferentiated retinal pigment epithelial cells are immunoreactive for vascular endothelial growth factor in surgically excised age-related macular degeneration-related choroidal neovascular membranes. Invest Ophthalmol Vis Sci 1996; 37 (5): 855–868.
Frank RN, Amin RH, Eliott D, Puklin JE, Abrams GW . Basic fibroblast growth factor and vascular endothelial growth factor are present in epiretinal and choroidal neovascular membranes. Am J Ophthalmol 1996; 122 (3): 393–403.
Oh H, Takagi H, Takagi C, Suzuma K, Otani A, Ishida K et al. The potential angiogenic role of macrophages in the formation of choroidal neovascular membranes. Invest Ophthalmol Vis Sci 1999; 40 (9): 1891–1898.
Reddy VM, Zamora RL, Kaplan HJ . Distribution of growth factors in subfoveal neovascular membranes in age-related macular degeneration and presumed ocular histoplasmosis syndrome. Am J Ophthalmol 1995; 120 (3): 291–301.
Bian ZM, Elner SG, Strieter RM, Kunkel SL, Lukacs NW, Elner VM . IL-4 potentiates IL-1beta- and TNF-alpha-stimulated IL-8 and MCP-1 protein production in human retinal pigment epithelial cells. Curr Eye Res 1999; 18 (5): 349–357.
Elner SG, Strieter RM, Elner VM, Rollins BJ, Del Monte MA, Kunkel SL . Monocyte chemotactic protein gene expression by cytokine-treated human retinal pigment epithelial cells. Lab Invest 1991; 64 (6): 819–825.
Elner VM, Strieter RM, Elner SG, Baggiolini M, Lindley I, Kunkel SL . Neutrophil chemotactic factor (IL-8) gene expression by cytokine-treated retinal pigment epithelial cells. Am J Pathol 1990; 136 (4): 745–750.
Otani A, Takagi H, Oh H, Koyama S, Matsumura M, Honda Y . Expressions of angiopoietins and Tie2 in human choroidal neovascular membranes. Invest Ophthalmol Vis Sci 1999; 40 (9): 1912–1920.
Jo N, Mailhos C, Ju M, Cheung E, Bradley J, Nishijima K et al. Inhibition of platelet-derived growth factor B signaling enhances the efficacy of anti-vascular endothelial growth factor therapy in multiple models of ocular neovascularization. Am J Pathol 2006; 168 (6): 2036–2053.
Lima e Silva R, Shen J, Hackett SF, Kachi S, Akiyama H, Kiuchi K et al. The SDF-1/CXCR4 ligand/receptor pair is an important contributor to several types of ocular neovascularization. FASEB J 2007; 21 (12): 3219–3230.
Semenza GL . HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol (1985) 2000; 88 (4): 1474–1480.
Wang GL, Jiang BH, Rue EA, Semenza GL . Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA 1995; 92 (12): 5510–5514.
Wang GL, Semenza GL . General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc Natl Acad Sci USA 1993; 90 (9): 4304–4308.
Campochiaro PA . Ocular neovascularization. J Mol Med (Berl) 2013; 91 (3): 311–321.
McLeod DS, Grebe R, Bhutto I, Merges C, Baba T, Lutty GA . Relationship between RPE and choriocapillaris in age-related macular degeneration. Invest Ophthalmol Vis Sci 2009; 50 (10): 4982–4991.
Mullins RF, Johnson MN, Faidley EA, Skeie JM, Huang J . Choriocapillaris vascular dropout related to density of drusen in human eyes with early age-related macular degeneration. Invest Ophthalmol Vis Sci 2011; 52 (3): 1606–1612.
Nasir MA, Sugino I, Zarbin MA . Decreased choriocapillaris perfusion following surgical excision of choroidal neovascular membranes in age-related macular degeneration. Br J Ophthalmol 1997; 81 (6): 481–489.
Sarks JP, Sarks SH, Killingsworth MC . Morphology of early choroidal neovascularisation in age-related macular degeneration: correlation with activity. Eye (Lond) 1997; 11 (Pt 4): 515–522.
Chandel NS, McClintock DS, Feliciano CE, Wood TM, Melendez JA, Rodriguez AM et al. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J Biol Chem 2000; 275 (33): 25130–25138.
Dong A, Xie B, Shen J, Yoshida T, Yokoi K, Hackett SF et al. Oxidative stress promotes ocular neovascularization. J Cell Physiol 2009; 219 (3): 544–552.
Olson N, van der Vliet A . Interactions between nitric oxide and hypoxia-inducible factor signaling pathways in inflammatory disease. Nitric Oxide 2011; 25 (2): 125–137.
Sengupta N, Caballero S, Mames RN, Butler JM, Scott EW, Grant MB . The role of adult bone marrow-derived stem cells in choroidal neovascularization. Invest Ophthalmol Vis Sci 2003; 44 (11): 4908–4913.
Fagiani E, Christofori G . Angiopoietins in angiogenesis. Cancer Lett 2013; 328 (1): 18–26.
Nambu H, Nambu R, Oshima Y, Hackett SF, Okoye G, Wiegand S et al. Angiopoietin 1 inhibits ocular neovascularization and breakdown of the blood-retinal barrier. Gene Ther 2004; 11 (10): 865–873.
Nambu H, Umeda N, Kachi S, Oshima Y, Akiyama H, Nambu R et al. Angiopoietin 1 prevents retinal detachment in an aggressive model of proliferative retinopathy, but has no effect on established neovascularization. J Cell Physiol 2005; 204 (1): 227–235.
Green WR, Enger C . Age-related macular degeneration histopathologic studies. The 1992 Lorenz E. Zimmerman Lecture. Ophthalmology 1993; 100 (10): 1519–1535.
Werner S, Grose R . Regulation of wound healing by growth factors and cytokines. Physiol Rev 2003; 83 (3): 835–870.
Schlingemann RO . Role of growth factors and the wound healing response in age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol 2004; 242 (1): 91–101.
Doucet C, Brouty-Boye D, Pottin-Clemenceau C, Canonica GW, Jasmin C, Azzarone B . Interleukin (IL) 4 and IL-13 act on human lung fibroblasts. Implication in asthma. J Clin Invest 1998; 101 (10): 2129–2139.
Gordon S . Alternative activation of macrophages. Nat Rev Immunol 2003; 3 (1): 23–35.
Wynn TA . Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol 2004; 4 (8): 583–594.
Juel HB, Faber C, Svendsen SG, Vallejo AN, Nissen MH . Inflammatory cytokines protect retinal pigment epithelial cells from oxidative stress-induced death. PLoS One 2013; 8 (5): e64619.
Cruz-Guilloty F, Saeed AM, Duffort S, Cano M, Ebrahimi KB, Ballmick A et al. T cells and macrophages responding to oxidative damage cooperate in pathogenesis of a mouse model of age-related macular degeneration. PLoS One 2014; 9 (2): e88201.
Faber C, Singh A, Kruger Falk M, Juel HB, Sorensen TL, Nissen MH . Age-related macular degeneration is associated with increased proportion of CD56(+) T cells in peripheral blood. Ophthalmology 2013; 120 (11): 2310–2316.
Juel HB, Kaestel C, Folkersen L, Faber C, Heegaard NH, Borup R et al. Retinal pigment epithelial cells upregulate expression of complement factors after co-culture with activated T cells. Exp Eye Res 2011; 92 (3): 180–188.
Sandler NG, Mentink-Kane MM, Cheever AW, Wynn TA . Global gene expression profiles during acute pathogen-induced pulmonary inflammation reveal divergent roles for Th1 and Th2 responses in tissue repair. J Immunol 2003; 171 (7): 3655–3667.
Gordon S, Taylor PR . Monocyte and macrophage heterogeneity. Nat Rev Immunol 2005; 5 (12): 953–964.
Mantovani A, Sica A, Locati M . Macrophage polarization comes of age. Immunity 2005; 23 (4): 344–346.
Armour A, Scott PG, Tredget EE . Cellular and molecular pathology of HTS: basis for treatment. Wound Repair Regen 2007; 15 (Suppl 1): S6–S17.
Fernando MR, Reyes JL, Iannuzzi J, Leung G, McKay DM . The pro-inflammatory cytokine, interleukin-6, enhances the polarization of alternatively activated macrophages. PLoS One 2014; 9 (4): e94188.
Erwig LP, Kluth DC, Walsh GM, Rees AJ . Initial cytokine exposure determines function of macrophages and renders them unresponsive to other cytokines. J Immunol 1998; 161 (4): 1983–1988.
Cuneo AA, Autieri MV . Expression and function of anti-inflammatory interleukins: the other side of the vascular response to injury. Curr Vasc Pharmacol 2009; 7 (3): 267–276.
Cao X, Shen D, Patel MM, Tuo J, Johnson TM, Olsen TW et al. Macrophage polarization in the maculae of age-related macular degeneration: a pilot study. Pathol Int 2011; 61 (9): 528–535.
Huang Y, Yuan J, Righi E, Kamoun WS, Ancukiewicz M, Nezivar J et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc Natl Acad Sci USA 2012; 109 (43): 17561–17566.
Osawa T, Tsuchida R, Muramatsu M, Shimamura T, Wang F, Suehiro J et al. Inhibition of histone demethylase JMJD1A improves anti-angiogenic therapy and reduces tumor-associated macrophages. Cancer Res 2013; 73 (10): 3019–3028.
Ambati J, Anand A, Fernandez S, Sakurai E, Lynn BC, Kuziel WA et al. An animal model of age-related macular degeneration in senescent Ccl-2- or Ccr-2-deficient mice. Nat Med 2003; 9 (11): 1390–1397.
Apte RS, Richter J, Herndon J, Ferguson TA . Macrophages inhibit neovascularization in a murine model of age-related macular degeneration. PLoS Med 2006; 3 (8): e310.
Cousins SW, Espinosa-Heidmann DG, Csaky KG . Monocyte activation in patients with age-related macular degeneration: a biomarker of risk for choroidal neovascularization? Arch Ophthalmol 2004; 122 (7): 1013–1018.
Espinosa-Heidmann DG, Suner IJ, Hernandez EP, Monroy D, Csaky KG, Cousins SW . Macrophage depletion diminishes lesion size and severity in experimental choroidal neovascularization. Invest Ophthalmol Vis Sci 2003; 44 (8): 3586–3592.
Soo C, Hu FY, Zhang X, Wang Y, Beanes SR, Lorenz HP et al. Differential expression of fibromodulin, a transforming growth factor-beta modulator, in fetal skin development and scarless repair. Am J Pathol 2000; 157 (2): 423–433.
Lee TY, Chin GS, Kim WJ, Chau D, Gittes GK, Longaker MT . Expression of transforming growth factor beta 1, 2, and 3 proteins in keloids. Ann Plast Surg 1999; 43 (2): 179–184.
Bock O, Yu H, Zitron S, Bayat A, Ferguson MW, Mrowietz U . Studies of transforming growth factors beta 1-3 and their receptors I and II in fibroblast of keloids and hypertrophic scars. Acta Derm Venereol 2005; 85 (3): 216–220.
Du S, Wang S, Wu Q, Hu J, Li T . Decorin inhibits angiogenic potential of choroid-retinal endothelial cells by downregulating hypoxia-induced Met, Rac1, HIF-1alpha and VEGF expression in cocultured retinal pigment epithelial cells. Exp Eye Res 2013; 116: 151–160.
Hagedorn M, Esser P, Wiedemann P, Heimann K . Tenascin and decorin in epiretinal membranes of proliferative vitreoretinopathy and proliferative diabetic retinopathy. Ger J Ophthalmol 1993; 2 (1): 28–31.
Inatani M, Tanihara H, Honjo M, Hangai M, Kresse H, Honda Y . Expression of proteoglycan decorin in neural retina. Invest Ophthalmol Vis Sci 1999; 40 (8): 1783–1791.
Kirwan RP, Wordinger RJ, Clark AF, O'Brien CJ . Differential global and extra-cellular matrix focused gene expression patterns between normal and glaucomatous human lamina cribrosa cells. Mol Vis 2009; 15: 76–88.
Nassar K, Luke J, Luke M, Kamal M, Abd El-Nabi E, Soliman M et al. The novel use of decorin in prevention of the development of proliferative vitreoretinopathy (PVR). Graefes Arch Clin Exp Ophthalmol 2011; 249 (11): 1649–1660.
Mohan RR, Tandon A, Sharma A, Cowden JW, Tovey JC . Significant inhibition of corneal scarring in vivo with tissue-selective, targeted AAV5 decorin gene therapy. Invest Ophthalmol Vis Sci 2011; 52 (7): 4833–4841.
Border WA, Noble NA, Yamamoto T, Harper JR, Yamaguchi Y, Pierschbacher MD et al. Natural inhibitor of transforming growth factor-beta protects against scarring in experimental kidney disease. Nature 1992; 360 (6402): 361–364.
Mohan RR, Tovey JC, Gupta R, Sharma A, Tandon A . Decorin biology, expression, function and therapy in the cornea. Curr Mol Med 2011; 11 (2): 110–128.
Yamaguchi Y, Mann DM, Ruoslahti E . Negative regulation of transforming growth factor-beta by the proteoglycan decorin. Nature 1990; 346 (6281): 281–284.
Sayani K, Dodd CM, Nedelec B, Shen YJ, Ghahary A, Tredget EE et al. Delayed appearance of decorin in healing burn scars. Histopathology 2000; 36 (3): 262–272.
Sidgwick GP, Bayat A . Extracellular matrix molecules implicated in hypertrophic and keloid scarring. J Eur Acad Dermatol Venereol 2012; 26 (2): 141–152.
Massova I, Kotra LP, Fridman R, Mobashery S . Matrix metalloproteinases: structures, evolution, and diversification. FASEB J 1998; 12 (12): 1075–1095.
Nagase H, Visse R, Murphy G . Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res 2006; 69 (3): 562–573.
Bride J, Viennet C, Lucarz-Bietry A, Humbert P . Indication of fibroblast apoptosis during the maturation of disc-shaped mechanically stressed collagen lattices. Arch Dermatol Res 2004; 295 (8-9): 312–317.
Kobayashi T, Liu X, Kim HJ, Kohyama T, Wen FQ, Abe S et al. TGF-beta1 and serum both stimulate contraction but differentially affect apoptosis in 3D collagen gels. Respir Res 2005; 6: 141.
Mazure A, Grierson I . In vitro studies of the contractility of cell types involved in proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci 1992; 33 (12): 3407–3416.
Akasaka Y, Fujita K, Ishikawa Y, Asuwa N, Inuzuka K, Ishihara M et al. Detection of apoptosis in keloids and a comparative study on apoptosis between keloids, hypertrophic scars, normal healed flat scars, and dermatofibroma. Wound Repair Regen 2001; 9 (6): 501–506.
Moulin V, Larochelle S, Langlois C, Thibault I, Lopez-Valle CA, Roy M . Normal skin wound and hypertrophic scar myofibroblasts have differential responses to apoptotic inductors. J Cell Physiol 2004; 198 (3): 350–358.
Nedelec B, Shankowsky H, Scott PG, Ghahary A, Tredget EE . Myofibroblasts and apoptosis in human hypertrophic scars: the effect of interferon-alpha2b. Surgery 2001; 130 (5): 798–808.
Quan TE, Cowper S, Wu SP, Bockenstedt LK, Bucala R . Circulating fibrocytes: collagen-secreting cells of the peripheral blood. Int J Biochem Cell Biol 2004; 36 (4): 598–606.
Mori L, Bellini A, Stacey MA, Schmidt M, Mattoli S . Fibrocytes contribute to the myofibroblast population in wounded skin and originate from the bone marrow. Exp Cell Res 2005; 304 (1): 81–90.
Wu Y, Wang J, Scott PG, Tredget EE . Bone marrow-derived stem cells in wound healing: a review. Wound Repair Regen 2007; 15 (Suppl 1): S18–S26.
Abe R, Donnelly SC, Peng T, Bucala R, Metz CN . Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol 2001; 166 (12): 7556–7562.
Yang L, Scott PG, Dodd C, Medina A, Jiao H, Shankowsky HA et al. Identification of fibrocytes in postburn hypertrophic scar. Wound Repair Regen 2005; 13 (4): 398–404.
Bissell MJ, Hall HG, Parry G . How does the extracellular matrix direct gene expression? J Theor Biol 1982; 99 (1): 31–68.
Clark RA . Basics of cutaneous wound repair. J Dermatol Surg Oncol 1993; 19 (8): 693–706.
Eckes B, Nischt R, Krieg T . Cell-matrix interactions in dermal repair and scarring. Fibrogenesis Tissue Repair 2010; 3: 4.
Herman IM . Extracellular matrix-cytoskeletal interactions in vascular cells. Tissue Cell 1987; 19 (1): 1–19.
Lee S, Zeiger A, Maloney JM, Kotecki M, Van Vliet KJ, Herman IM . Pericyte actomyosin-mediated contraction at the cell-material interface can modulate the microvascular niche. J Phys Condens Matter 2010; 22 (19): 194115.
Raghow R . The role of extracellular matrix in postinflammatory wound healing and fibrosis. FASEB J 1994; 8 (11): 823–831.
Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA . Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol 2002; 3 (5): 349–363.
Young WC, Herman IM . Extracellular matrix modulation of endothelial cell shape and motility following injury in vitro. J Cell Sci 1985; 73: 19–32.
Berrier AL, Yamada KM . Cell-matrix adhesion. J Cell Physiol 2007; 213 (3): 565–573.
Hynes RO . The extracellular matrix: not just pretty fibrils. Science 2009; 326 (5957): 1216–1219.
Schultz GS, Davidson JM, Kirsner RS, Bornstein P, Herman IM . Dynamic reciprocity in the wound microenvironment. Wound Repair Regen 2011; 19 (2): 134–148.
Busik JV, Tikhonenko M, Bhatwadekar A, Opreanu M, Yakubova N, Caballero S et al. Diabetic retinopathy is associated with bone marrow neuropathy and a depressed peripheral clock. J Exp Med 2009; 206 (13): 2897–2906.
Acknowledgements
I thank Paul Quinlan (KO-OP Media Design) for his expertise with the illustrations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no conflict of interest.
Rights and permissions
About this article
Cite this article
Kent, D. The stereotypical molecular cascade in neovascular age-related macular degeneration: the role of dynamic reciprocity. Eye 29, 1416–1426 (2015). https://doi.org/10.1038/eye.2015.140
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2015.140