Abstract
Aims
To report the outcomes of the use of intracameral bevacizumab for iris neovascularization occurring after silicone oil (SO) removal in eyes undergoing vitreoretinal surgery (VRS).
Material and Methods
This study included 12 eyes that had iris neovascularization after SO removal. The clinical outcomes of 12 eyes after intravitreal bevacizumab injection were reviewed.
Results
There were eight men and four women with an average age of 41.58±12.68 years. All eyes had VRS for various vitreoretinal diseases. After the mean follow-up period of 9.7±5.3 months, SO removal was performed. Then, the patients were followed for more than 2 months and detailed retinal examinations and intraocular pressure (IOP) were normal during this period, but rubeosis iridis (RI) developed. RI was treated with 1 dose of 1.25 mg bevacizumab into the anterior chamber. After a mean follow-up period of 4.8±2.2 months, the regression of iris neovacularization was detected and IOP was below 21 mmHg in all eyes.
Conclusion
Anterior segment neovascularization (ASNV) may develop through various mechanisms in patients with VRS after SO removal, and anterior chamber injection of bevacizumab may lead to regression of ASNV.
Similar content being viewed by others
Introduction
Anterior segment neovascularization (ASNV) has been one of the devastating complications after vitreoretinal surgery (VRS) with and without silicone oil (SO). Several studies have reported the formation of rubeosis iridis (RI) after VRS and the precise mechanism of the development of ASNV has been still unclear.1, 2, 3, 4, 5, 6 ASVN may lead to inevitable ocular complications, such as painful neovascular glaucoma.5, 6 Regressive approaches for ASNV, including antivascular endothelial growth factor (VEGF), may prevent the development of devastating consequences.
Bevacizumab (Avastin) is an anti-VEGF agent and inhibits VEGF. It produces stabilization or regression of neovascular activity and vascular permeability.7 Intravitreal administration of bevacizumab has been commonly used in various ocular diseases, such as diabetic retinopathy, age-related macular disease, and vein occlusion among others.8 Recently, several studies have been reported regarding the use of intracameral or intravitreal bevacizumab for the management of neovascular glaucoma and RI.9, 10
In this study, we report regression of RI after the injection of intracameral bevacizumab in eyes with VRS after SO removal.
Materials and methods
Twelve eyes of 12 patients who had iris neovascularization after SO removal were treated with intracameral bevacizumab injection, and clinical outcomes were reviewed in this study. This study was approved by the institutional ethics committee. Written informed consent was obtained from all participants before the enrolment of the participants in the study after the nature of the intervention was clearly explained.
After obtaining detailed medical histories, the patients underwent pre-injection examination, including corrected visual acuity measurements with Snellen chart, the measurement of intraocular pressure (IOP) with applanation tonometer, biomicroscopic anterior chamber, and dilated fundus examinations.
Exclusion criteria were thromboembolic events (including myocardial infarction or cerebral vascular events), major surgery within the previous 3 months, uncontrolled hypertension, and known coagulation abnormalities.
All intravitreal injections of 1.25 mg bevacizumab were performed using proparacaine (Alcaine; Alcon Laboratories Inc., Fort Worth, TX, USA) under sterile conditions (eyelid speculum, eye drapes, and povidone-iodine). Bevacizumab (1.25 mg) was injected into the anterior chamber using a 30-gauge needle through the limbus. The patients received topical 0.3% ciprofloxacin four times daily after the procedure.
Postinjection follow-up examinations were performed weekly in the first month and monthly thereafter. Postinjection evaluations including the measurement of IOP with applanation tonometer, detailed anterior segment, and dilated fundus examinations were recorded.
Results
There were eight men and four women with an average age of 41.58±12.68 years. Three eyes had proliferative diabetic retinopathy (PDR with epimacular and/or epiretinal fibrovascular membranes and no tractional detachment), one eye had PDR with thick fibrovascular membranes and tractional retinal detachment (TRD), two eyes had PDR with only thin fibrovascular proliferation (no tractional detachment) and vitreous haemorrhage (VH), one eye had TRD (not diabetic), four eyes had giant retinal tear (GRT) and RD, and one eye had eales disease and VH, and all eyes underwent VRS with SO injection (1000cs or 5000cs). Three eyes were pseudophakic and nine eyes were phakic. IOP and anterior segment examination findings were normal in all eyes before VRS.
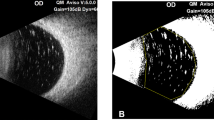
There was no complication during the mean follow-up period of 9.7±5.3 months after VRS. Then, SO removal was performed and the patients were followed for more than 2 months. After SO removal, detailed retinal examinations and IOP were normal, but the development of RI was observed. RI was detected by slit lamp anterior segment examination. It was treated with 1 dose of 1.25 mg bevacizumab into the anterior chamber and the patients were followed for the mean period of 4.8±2.2 months (Table 1). At the last examination, the regression of RI was detected by slit lamp anterior segment examination and IOP was below 21 mmHg in all eyes. No intraocular inflammation and complication were observed during the follow-up period after injection.
Discussion
Silicone oil has been commonly used in complicated vitreoretinal diseases and it has been considered useful in preventing the onset or aggravation of RI, and neovascular response has accelerated after SO removal in eyes with VRS.11, 12, 13, 14 In this study, we observed the development of RI in eyes undergoing VRS after SO removal and reported regression of RI with the injection of intracameral bevacizumab.
Several mechanisms have been proposed to explain the development of RI after SO removal. Although complete retinal laser photocoagulation has already been performed before and/or during operations in eyes with PDR, failure to regulate blood glucose and persistent ischaemia may increase the likelihood of anterior and posterior segment neovascularization after SO removal. In addition, disruption of the blood–retina barrier may occur in the eyes undergoing vitrectomy, and this situation may contribute to the development of neovascularization.1, 3 When the inhibitory effect of SO disappears, neovascularization in either the anterior or posterior segment may increase.
An alternative mechanism having a role in the development of RI may be a regression in the release of the aqueous humour. The association of hypotony with RI has been reported in several studies.15 When SO is kept in the vitreous cavity for a long time, it may have either mechanical or toxic effects, which may lead to a considerable regression in the excretion of the aqueous humour. It should be kept in mind that there is a tendency to hypotony in such cases.1, 16, 17 When the inhibitory effect of SO disappears, ischaemia may become more marked.
Scleral buckling has been used in some cases of complicated retinal detachment2 and it has also been required to avoid retinal shortening in some cases of RD with GRT.18 Such practices are also known to cause ischaemia in the anterior segment and neovascularization, and ischaemia may increase markedly following SO removal.2 In addition, if the anterior part of the tear is not excised in eyes with giant tears, an atrophied anterior piece of tear may be forced towards the anterior part by intravitreal SO and a marked proliferation between the atrophied piece and the ciliary body may occur.19 Both adhesions due to this proliferation and excess aqueous humour resorbed through the large choroidal opening, and also, mechanical and toxic effects of SO cause reduced amount of aqueous humour, and a subsequent ischaemia can induce neovascularization following SO removal.
In our study, VRS included complete retinal laser photocoagulation, scleral buckling, and SO injection in eyes with PDR and/or TRD, and in eyes with RD and GRT, VRS with SO injection were combined with scleral buckling. Other eyes underwent VRS with SO injection. Retinal detachment and other postoperative complications did not occur during the follow-up period with SO, but we observed RI in these eyes after SO removal and we thought that depending on the combination of abovementioned conditions, a marked ASNV appeared in our series. We used intracameral bevacizumab to reduce RI in these eyes.
Intracameral bevacizumab prevents neovascularization through several mechanisms. Bevacizumab binds and inhibits all the biologically active form of VEGF and decreases neovascularization, and it also has a regulatory effect on the blood–retina barrier disrupted after SO removal in the eyes undergoing vitrectomy. In addition, intracameral bevacizumab decreases ischaemia in the posterior segment, which eliminates ASNV.8 Regression of the iris neovascularizations and neovascular glaucoma have been reported after the injection of bevacizumab in several studies.9, 10 Grisanti et al9 have reported the regression of iris neovascularization after intracameral bevacizumab injection in six eyes of three patients with PDR (two patients) and ischaemic vein occlusion (one patient) within 4 weeks of follow-up. Qureshi et al10 treated neovascular glaucoma secondary to ischaemic central retinal vein occlusion in two patients using intracameral bevacizumab injection. Falavarjani et al6 have reported the intrasilicone injection of bevacizumab in order to treat neovascular glaucoma after vitrectomy in five eyes with diabetic retinopathy. In their study, they observed complete regression of RI within 7 days after injection and they concluded that bevacizumab injection was effective in the management of RI after vitrectomy.6
In this study, we used intracameral bevacizumab injection to treat ASNV. We observed regression of neovascularization within the follow-up period and there was no recurrence after 4.8 months follow up. In this study, the follow-up period was short after bevacizumab injection. However, as it is difficult to achieve systemic regulation of PDR, long-term outcomes of avastin injection can be misleading. In fact, short-term outcomes of avastin injection can better indicate the duration of avastin efficacy.
In conclusion, our observations indicate that secondary mechanisms may lead to neovascular activity in eyes with VRS after SO removal. Intracameral bevacizumab can prevent neovascularization of the anterior segment after SO removal. Early prevention of neovascular activity in the anterior segment should be the aim of optimal care for eyes with VRS.

References
Comaratta MR, Chang S, Sparrow J . Iris neovascularization in proliferative vitreoretinopathy. Ophthalmology 1992; 99: 898–905.
Cohen S, Kremer I, Yassur Y, Ben-Sira I . Peripheral retinal neovascularization and rubeosis iridis after a bilateral circular buckling operation. Ann Ophthalmol 1988; 20: 153–156.
Barile GR, Chang S, Horowitz JD, Reppucci VS, Schiff WM, Wong DT . Neovascular complications associated with rubeosis iridis and peripheral retinal detachment after retinal detachment surgery. Am J Ophthalmol 1998; 126: 379–389.
Oldendoerp J, Spitznas M . Factors influencing the results of vitreous surgery in diabetic retinopathy. I. Iris rubeosis and/or active neovascularization at the fundus. Graefes Arch Clin Exp Ophthalmol 1989; 227: 1–8.
Wand M, Madigan JC, Gaudio AR, Sorokanich S . Neovascular glaucoma following pars plana vitrectomy for complications of diabetic retinopathy. Ophthalmic Surg 1990; 21: 113–118.
Falavarjani KG, Modarres M, Nazari H . Therapeutic effect of bevacizumab injected into the silicone oil in eyes with neovascular glaucoma after vitrectomy for advanced diabetic retinopathy. Eye 2009. e-pub ahead of print 1 May 2009. doi:10.1038/eye.2009.94.
Ferrara N, Hillan KJ, Novotny W . Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem Biophys Res Commun 2005; 333: 328–335.
Lynch SS, Cheng CM . Bevacizumab for neovascular ocular diseases. Ann Pharmacother 2007; 41: 614–625.
Grisanti S, Biester S, Peters S, Tatar O, Ziemssen F, Bartz-Schmidt KU, Tuebingen Bevacizumab Study Group. Intracameral bevacizumab for iris rubeosis. Am J Ophthalmol 2006; 142: 158–160.
Qureshi K, Kashani S, Kelly SP . Intracameral bevacizumab for rubeotic glaucoma secondary to retinal vein occlusion. Int Ophthalmol 2008. E-pub ahead of print 14 September 2008.
McCuen 2nd BW, Rinkoff JS . Silicone oil for progressive anterior ocular neovascularization after failed diabetic vitrectomy. Arch Ophthalmol 1989; 107: 677–682.
Federman JL, Schubert HD . Complications associated with the use of silicone oil in 150 eyes after retina-vitreous surgery. Ophthalmology 1988; 95: 870–876.
Brourman ND, Blumenkranz MS, Cox MS, Trese MT . Silicone oil for the treatment of severe proliferative diabetic retinopathy. Ophthalmology 1989; 96: 759–764.
Azzolini C, Brancato R, Camesasca FI, Codenotti M . Influence of silicone oil on iris microangiopathy in diabetic vitrectomized eyes. Ophthalmology 1993; 100: 1152–1158.
Peyman GA, Raichand M, Juarez CP, Reinglass S, John E . Hypotony and experimental rubeosis iridis in primate eyes. A clinicopathologic study. Graefes Arch Clin Exp Ophthalmol 1986; 224: 435–442.
Barr CC, Lai MY, Lean JS, Linton KL, Trese M, Abrams G et al. Postoperative intraocular pressure abnormalities in the Silicone Study. Silicone Study Report 4. Ophthalmology 1993; 100: 1629–1635.
Burk LL, Shields MB, Proia AD, McCuen 2nd BW . Intraocular pressure following intravitreal silicone oil injection. Ophthalmic Surg 1988; 19: 565–569.
Holland PM, Smith TR . Broad scleral buckle in the management of retinal detachments with giant tears. Am J Ophthalmol 1977; 83: 518–525.
Haut J, Larricart P, van Effenterre G . Localized retinectomy indications in the treatment and prevention of retinal detachment. Ophthalmologica 1984; 188: 212–215.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Batman, C., Ozdamar, Y. The effect of bevacizumab for anterior segment neovascularization after silicone oil removal in eyes with previous vitreoretinal surgery. Eye 24, 1243–1246 (2010). https://doi.org/10.1038/eye.2009.304
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2009.304
Keywords
This article is cited by
-
Mutations at Tyrosine 88, Lysine 92 and Tyrosine 470 of Human Dopamine Transporter Result in an Attenuation of HIV-1 Tat-Induced Inhibition of Dopamine Transport
Journal of Neuroimmune Pharmacology (2015)