Abstract
Purpose
To describe the use of the second-generation QuantiFERON-TB Gold (QFT-G) test in a series of patients in an ophthalmic practice.
Methods
The charts of all patients who had QFT-G tests ordered by Mayo Clinic ophthalmologists in the past 3 years were reviewed.
Results
A total of 27 QFT-G tests were ordered. Thirteen (48%) tests were negative, six (22%) were indeterminate, two (7%) tests were re-ordered after a lab accident or an improper cancellation, four (15%) were positive and represented infection, and two (7%) were positive but negative when re-tested. Of the four truly positive cases, three were treated for tuberculosis (TB): one had tuberculous iritis, one had retinal vasculitis and haemorrhage, and one had asymptomatic TB but was on immunosuppressive therapy. The fourth patient had previously been treated for latent infection.
Conclusions
In a series of selected patients with uveitis, the QFT-G test was able to detect TB infection in 15% of the patients, though it does not differentiate between active and latent TB infection. QFT-G should be considered in place of purified protein derivative testing in those with uveitis that have had prior BCG vaccination and in immunocompromised patients. Patients with a positive QFT-G, but who have little risk for TB infection and a negative systemic work-up, should be re-tested.
Similar content being viewed by others
Introduction
Tuberculosis (TB), an infection caused by Mycobacterium tuberculosis, can involve any part of the eye with or without other primary foci in the body.1 As ocular lesions are often difficult to culture, ocular TB is suspected when concomitant TB is present elsewhere in the body. Currently, detection of latent TB is accomplished by tuberculin skin testing (TST) with purified protein derivative (PPD) or recombinant purified protein derivatives; intradermal injections of these extracts into persons infected with M. tuberculosis elicit a delayed-type hypersensitivity maximally between 48 and 72 h. The test is susceptible to placement errors, reading errors, false negatives in anergic patients, false positives in BCG-vaccinated patients, the booster phenomenon where repeated testing induces a positive result, and non-compliance as it requires two visits to the physician.2
A recently released assay, the QuantiFERON-TB Gold (QFT-G) blood test (Cellestis Limited, Carnegie, Victoria, Australia), utilizes the principle that previously sensitized T cells will produce interferon (IFN)-γ upon re-exposure. When serum from patients is exposed to TB-specific antigens, high levels of IFN-γ are detected by enzyme-linked immunosorbent assay and are indicative of TB infection.3 The assay is run along with a positive control well, where T cells in the sample are non-specifically stimulated; if IFN-γ is not detected in this well, the result of the assay is indeterminate. Like the TST, the QFT-G test does not distinguish between active or latent TB infection; however, this assay does appear to be sensitive and specific even in the presence of prior BCG vaccination. We report on results of the use of this test by ophthalmologists at the Mayo Clinic.
Materials and methods
Following approval by the Mayo Clinic Institutional Review Board, Mayo Clinic electronic medical record databases were searched for all orders for the QFT-G test made by ophthalmologists from March 2004 to March 2007. The patients’ charts were reviewed for demographics, reason for referral, previous TB status, medications, eye examination findings, QFT-G results, final diagnosis, treatment, systemic patient conditions, and status at last eye exam. The assay used at the Mayo Clinic is the second generation of the QuantiFERON test that was approved by the Food and Drug Administration in 2005. We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during this research.
Results
A total of 27 QFT-G tests were ordered. Thirteen (48%) tests were negative, six (22%) were indeterminate, two (7%) tests were re-ordered after a lab accident or an improper cancellation, four (15%) were positive and represented infection, and two (7%) were positive and then negative when re-tested. The patients with negative and indeterminate results are summarized in the Supplementary Table.
The phenomenon of IFN-γ assays first producing a positive result and then a negative result has been termed reversion. For one patient with reversion, the QFT-G test was initially ordered after the patient developed uveitis after a bevacizumab injection for age-related macular degeneration. Subsequent work-up with chest contrast tomography was negative and work-up for TB was negative by a primary care physician. When the patient was re-tested, 2 months after initial testing, the QFT-G test was negative. The second patient with reversion was first tested after iris neovascularization secondary to corneal erosion. The ulcer and neovascularization resolved after treatment with prednisolone acetate, 1% atropine, gentamicin, and 0.5% ciprofloxacin. Since the patient did not have any systemic symptoms, abnormalities on chest X-ray (CXR), or recurrence of ocular inflammation, she was re-tested after 11 months and found to be negative.
The four positive cases are summarized in Table 1. Of the four positive cases that represented infection, three were treated for TB; one had tuberculous uveitis, one had retinal vasculitis and haemorrhage, and one had asymptomatic TB but was on immunosuppressive therapy. The fourth patient had already been treated with a 9-month course of isoniazid after emigrating from Vietnam around 15 years prior. Since his VDRL was positive, his iris neovascularization was attributed to neurosyphilis and not TB. The positive cases where treatment was initiated are described in the case reports below.
Case reports
Patient 1
A 23-year-old Somali man was referred because of constant pain in both eyes for 2 years, right more than left. The patient had a positive PPD skin test on a refugee physical 4 months prior. However, he had no systemic symptoms and his CXR was unremarkable, so his positive PPD was attributed to BCG vaccination. The patient was not encouraged to complete a 6- to 9-month course of isoniazid prescribed for him. At that time, the patient had also been treated by his local ophthalmologist with prednisolone acetate 1% eyedrops for bilateral granulomatous uveitis.
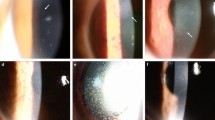
On examination at the Mayo Clinic, visual acuity in the right eye was 7/200 and 20/20 in the left eye. The right eye findings included multiple inferior pigmented keratic precipitates (KP) on the cornea, cells in the anterior chamber, Busacca and Koeppe nodules on the iris, pigment on the lens, 3+ diffuse posterior subcapsular cataract (PSC), and multiple punched out lesions in the retina. The left eye findings included KP, pigment on the lens, and inactive choroidal lesions. CXR, QFT-G, and HIV tests were ordered, and only the QFT-G was positive. The diagnosis of TB granulomatous panuveitis was made.
The patient was advised to take the isoniazid to treat the TB, and continue prednisolone acetate 1% eyedrops. After 2 months, the iris nodules were less apparent, but active inflammation was still present and the frequency of prednisolone acetate 1% eyedrops was increased. At his most recent appointment, 9 months after his initial referral evaluation, the iris nodules had disappeared and the fundus lesions appeared inactive; visual acuity OD was still 20/200, but may have been secondarily reduced due to a steroid-induced cataract. At follow-up, 9 months after initial presentation, the vision in the right and left eyes was 20/300 and 20/20, respectively. No conjunctival infection was present and no active uveitis was present. Choroidal scars as well as a dense PSC OD were present. After 2 months, he was evaluated for cataract surgery and his vision in his right eye was 20/250 pinholed to 20/25. He is scheduled to undergo cataract surgery soon.
Patient 2
A 33-year-old man was referred for complaints of a moon-shaped black spot in his peripheral vision and a suspected retinal astrocytoma OS. His right eye had been enucleated in 2005 for a suspected astrocytic hamartoma; significant medical conditions include Crohn's disease medically controlled since age 4 with infliximab and azathioprine.
At his initial visit, best-corrected visual acuity (BCVA) OS was 20/50 and examination revealed a peripheral acquired retinal hemangioma with cells in the vitreous and a PSC. He underwent extensive testing because the underlying diagnosis was uncertain. Complete blood count (CBC), erythrocyte sedimentation rate (ESR), alanine transaminase, lupus, leptospira, and syphilis serologies were all negative; the QFT-G test, anti-neutrophilic cytoplasmic antibodies were positive and angiotensin-converting enzyme level was slightly elevated. Chest computed tomography (CT) scan also demonstrated a 2–3 mm nodule in the right upper lobe. Genetic testing demonstrated three polymorphic variants in NOD2/CARD 15, and the diagnosis of Blau syndrome was made. Three sputum cultures were negative for M. tuberculosis. Due to changes in his CT scan of the chest, the positive QFT-G test, and his long-term immunosuppressive treatment for his Blau syndrome, he was begun on treatment for latent TB with isoniazid and pyridoxine for 30 days.
Patient 3
A 25-year-old male corrections officer was referred for evaluation of uveitis. Three months earlier, he had noticed the onset of blurry vision, floaters, and dark spots OU, and his local ophthalmologist diagnosed him with retinal vasculitis and started him on prednisone 60 mg per day orally for a month. His corticosteroids were tapered and he subsequently developed bilateral retinal neovascularization and vitreous haemorrhages for which he was treated with panretinal photocoagulation. His medical history was significant for idiopathic recurring seizures, controlled with topiramate. His most recent PPD test was negative in 2001.
At his referral examination, visual acuity was 20/200 OD and 20/25 OS. Examination of the right eye was remarkable for vitreous haemorrhage. In the left eye, there was a mild vitreous haemorrhage, and active retinal neovascularization elsewhere. There was severe retinal vasculitis OU with capillary non-perfusion and neovascularization. Other than a leukocytosis most likely secondary to systemic corticosteroids and a positive QFT-G result, labs including protein electrophoresis, creatinine, CBC, ESR, anti-nuclear antibody, anti-cyclic citrullinated peptide antibodies, anti-myeloperoxidase antibodies, cryoglobulins, HIV, syphilis, and Lyme serologies were negative; urine analysis and magnetic resonance imaging (MRI) of the head were also negative. Eale's disease was suspected. The infectious disease service recommended treatment of latent TB infection with a four-drug TB regimen of isoniazid (300 mg daily), rifampin (300 mg daily), pyrazinamide (15 mg per kg daily), and ethambutol (15 mg per kg daily) because the patient would be immunosuppressed for his ocular condition. The left eye was treated with pars plana vitrectomy and endolaser. A sample of vitreous was tested for TB and was negative.
Discussion
A total of 27 QFT-G tests were ordered by ophthalmologists at the Mayo Clinic in the past 3 years. Thirteen (48%) tests were negative, two (7%) were re-tests, two (7%) reverted to negative on subsequent testing, six (22%) were indeterminate, and four (15%) were positive.
The second-generation QFT-G test was approved by the US Food and Drug Administration in May 2005, and guidelines for its use were released by the Centers for Disease Control (CDC) in December of the same year.3 The major advantage of the QFT-G test is its increased specificity for detecting TB infection. Mori et al4 demonstrated this in 216 BCG-vaccinated patients with no risk for TB infection; the QFT-G test was negative in 98% of patients, whereas the TST was negative in only 35% of them. In culture-confirmed active TB, the sensitivity of the QFT-G test can be calculated and compared to the TST. A recent meta-analysis that pooled the results of nine studies calculated the sensitivity of the second-generation QFT-G in patients with active TB to be 80% (95% CI: 73–87%), whereas the pooled TST sensitivity from 14 studies was 71% (65–74%).5 Thus, it is important to realize that even a negative QFT-G does not rule out active TB disease. For latent tuberculosis infection (LTBI), there is no gold standard for detection; thus, it is impossible to calculate the exact sensitivity, positive predictive value, or negative predictive value of the QFT-G or the TST for detecting LTBI. Instead, investigators compare the two tests by correlating risk of TB exposure to the result of each test. In a study of 203 HIV-infected inner city patients, Jones and colleagues6 determined that the QFT-G results were more likely to be associated with risk factors for LTBI compared to the TST.
Mazurek et al7 compared TST with QFT-G in 1226 adults at five United States universities and found agreement of the tests in 83.1%, with discordance in BCG-vaccinated or MAC-exposed individuals where the skin test was positive and QFT-G negative. In our study, three patients had been previously tested with the TST; the QFT-G result correlated with the TST result in two of three cases. Patient 3 had a previously negative TST in 2001 and a positive QFT-G when tested at the Mayo Clinic in 2005; considering his occupation as a corrections officer, it is logical to assume that this change represents a true conversion and not a false-negative TST. Even in a case of a concordant result, the QFT-G result was helpful. Although the patient in case report 1 had a positive PPD, the patient had a normal CXR and no systemic symptoms so the TST result was attributed to BCG vaccination. He was not encouraged to finish his course of isoniazid, and his granulomatous uveitis was attributed to possible sarcoid and not TB.
In immunocompromised patients with culture-confirmed TB, Kobashi et al8 concluded that the QFT-G was a more useful diagnostic method than the TST as the QFT-G test was positive in 72% of cases whereas the TST in only 50%. They reported that 31% of patients with TB disease were QFT-G positive and TST negative, whereas none were QFT-G negative and TST positive.8 In our study, there were four patients on immunosuppressive medications: two tested QFT-G positive, one negative, and one indeterminate. There were no patients with HIV infection or lymphopenic disorders. As demonstrated in the second case report, identifying immunocompromised patients with latent infection by QFT-G was helpful since TB treatment could be initiated to protect the patient from developing active disease.
A relatively high percentage (22%) of the QFT-G tests yielded an indeterminate result. Ferrara et al2, in a series of hospitalized patients, found indeterminate results in 21% of 318 tests when the positive control test failed; this occurred more often in immunosuppressed patients. In our study, one (17%) of the six patients with indeterminate results was on immunosuppressive therapy at the time of the test. The high rate of indeterminate results was more likely the result of improper collection tubes; after switching collection tubes in May 2007, the Mayo Clinic laboratory noted that the rate of indeterminate results decreased from around 20% to approximately 5%.
Reversion was noted in 7% of our series, as two patients first tested positive by QFT-G and then negative. Few studies have investigated this phenomenon to date. Pai et al9 performed serial testing of QFT-G and TST in 216 medical care workers in India and found reversion after 18 months in 24% of patients who were initially QFT-G positive. Reversions occurred more often in patients with IFN responses close to the cut-off threshold and in patients with discordant QFT and TST results. Thus, reversion was concluded to be the result of a suboptimal threshold between a positive and negative result. According to studies conducted by the manufacturer on replicate serum samples from controlled patients, the test-related coefficient of variation for the QFT-G was 8.7%. Neither values of IFN-γ release nor TST results were available in our patients for analysis.
Currently, the CDC recommends that the QFT-G test be used in all cases where TST is currently being used. Compared to the TST, the QFT-G test requires only a single patient visit, is less likely to result in a false positive in BCG-vaccinated patients, and is less susceptible to reader bias and the booster phenomenon. For ophthalmologists it can be useful in the work-up of any observed inflammatory conditions to more quickly and accurately rule in or rule out TB infection. The results of our study and others suggest that the QFT-G is especially useful in BCG-vaccinated and immunocompromised patients. Although the QFT-G does represent an important advance in the diagnosis of TB, it is important to realize that similar to the TST the QFT-G does not distinguish between latent and active TB infection, nor does a negative QFT-G rule out TB infection. The QFT-G result should be considered in the context of the clinical picture. Ophthalmologists should be wary about a false-positive QFT-G result in patients with no risk factors for TB development, a negative work-up for systemic disease, another established cause for inflammation, and no recurrence of ocular symptoms; in these cases, the test should be repeated and the test may revert to negative.
References
Thompson MJ, Albert DM . Ocular tuberculosis. Arch Ophthalmol 2005; 123: 844–849.
Ferrara G, Losi M, Meacci M, Meccugni B, Piro R, Roversi P et al Routine hospital use of a new commercial whole blood interferon-gamma assay for the diagnosis of tuberculosis infection. Am J Respir Crit Care Med 2005; 172: 631–635.
Mazurek GH, Jereb J, Lobue P, Iademarco MF, Metchock B, Vernon A . Guidelines for using the QuantiFERON-TB Gold test for detecting Mycobacterium tuberculosis infection, United States. MMWR Recomm Rep 2005; 54: 49–55.
Mori T, Sakatani M, Yamagishi F, Takashima T, Kawabe Y, Nagao K et al Specific detection of tuberculosis infection: an interferon-gamma-based assay using new antigens. Am J Respir Crit Care Med 2004; 170: 59–64.
Menzies D . Interpretation of repeated tuberculin tests. Boosting, conversion, and reversion. Am J Respir Crit Care Med 1999; 159: 15–21.
Jones S, de Gijsel D, Wallach FR, Gurtman AC, Shi Q, Sacks H. Utility of QuantiFERON-TB Gold in-tube testing for latent TB infection in HIV-infected individuals. Int J Tuberc Lund Dis 2007; 11: 1190–1195.
Mazurek GH, LoBue PA, Daley CL, Bernardo J, Lardizabal AA, Bishai WR et al Comparison of a whole-blood interferon gamma assay with tuberculin skin testing for detecting latent Mycobacterium tuberculosis infection. JAMA 2001; 286: 1740–1747.
Kobashi Y, Mouri K, Obase Y, Fukuda M, Miyashita N, Oka M . Clinical evaluation of QuantiFERON TB-2G test for immunocompromised patients. Eur Respir J 2007; 30: 945–950.
Pai M, Joshi R, Dogra S, Mendiratta DK, Narang P, Kalantri S et al Serial testing of health care workers for tuberculosis using interferon-gamma assay. Am J Respir Crit Care Med 2006; 174: 349–355.
Acknowledgements
We thank Elaine Beito and Dr Robin Molella for their helpful comments. This study was supported by an unrestricted grant from Research to Prevent Blindness Inc., New York, NY and the Mayo Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors indicate no financial conflict of interest.
Contributions of authors: Involved in the design and conduct of the study, collection, management, analysis and interpretation of data, and preparation, review and approval of the manuscript and references (SI, SJB, JSP); involved in the design and conduct of the study (DCH, LJF, GTT, SRB, NSF); The study protocol was approved by the Mayo Institutional Review Board and conforms to HIPAA requirements. As a retrospective analysis, informed consent was not required from participants. However, in conformity with Minnesota state law, we did not include in this study any patients who have refused to allow their medical records reviewed for the purpose of medical research.
Supplementary Information accompanies the paper on Eye website (http://www.nature.com/eye)
Supplementary information
Rights and permissions
About this article
Cite this article
Itty, S., Bakri, S., Pulido, J. et al. Initial results of QuantiFERON-TB Gold testing in patients with uveitis. Eye 23, 904–909 (2009). https://doi.org/10.1038/eye.2008.115
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2008.115
Keywords
This article is cited by
-
Presumed ocular tuberculosis in the United Kingdom: a British Ophthalmological Surveillance Unit (BOSU) study
Eye (2020)
-
Structural changes of the choroid in sarcoid- and tuberculosis-related granulomatous uveitis
Eye (2015)
-
Clinical presentation, treatment, and outcomes in presumed intraocular tuberculosis: experience from Newcastle upon Tyne, UK
Eye (2013)
-
Utility of QuantiFERON®-TB Gold test in diagnosis and management of suspected tubercular uveitis in India
International Ophthalmology (2012)
-
The value of an immune response to Mycobacterium tuberculosis in patients with chronic posterior uveitides revisited: utility of the new IGRAs
Eye (2010)