Abstract
The direct differentiation of hepatocytes from bone marrow cells remains controversial. Several mechanisms, including transdifferentiation and cell fusion, have been proposed for this phenomenon, although direct visualization of the process and the underlying mechanisms have not been reported. In this study, we established an efficient in vitro culture method for differentiation of functioning hepatocytes from murine lineage-negative bone marrow cells. These cells reduced liver damage and incorporated into hepatic parenchyma in two independent hepatic injury models. Our simple and efficient in vitro protocol for endodermal precursor cell survival and expansion enabled us to identify these cells as existing in Sca1+ subpopulations of lineage-negative bone marrow cells. The endodermal precursor cells followed a sequential developmental pathway that included endodermal cells and hepatocyte precursor cells, which indicates that lineage-negative bone marrow cells contain more diverse multipotent stem cells than considered previously. The presence of equivalent endodermal precursor populations in human bone marrow would facilitate the development of these cells into an effective treatment modality for chronic liver diseases.
Similar content being viewed by others
Introduction
Cell-based therapies for hepatic failure offer an alternative to organ transplantation, which is not widely applicable to the majority of patients due to the lack of donor organs, immunological rejection and recurrence of original disease that often compromise long-term recipient survival.1, 2, 3 As embryonic and equivalent pluripotent stem cells have an inherent limitation of in vivo tumorigenicity,4 the generation of functioning hepatocytes from adult stem cells is the top priority in the treatment of hepatic failure.5 Bone marrow is an important source of adult stem cells, and two approaches to hepatocyte differentiation have been developed. In the first approach, hepatocytes are differentiated directly from bone marrow cells,6, 7, 8, 9, 10, 11, 12 and in the second, the establishment of multipotent stem cells is extended in vitro to allow hepatocyte differentiation.13, 14, 15, 16, 17
Two eminent research groups had documented hepatocyte differentiation from bone marrow cells by determining that KTLS (c-KithiThyloLin−Sca1+) hematopoietic stem cells (HSCs), but not c-Kit−, Sca1− and lineage-positive (Lin+) cells, differentiated into hepatocyte-like cells in a FAH−/− (fumarylacetoacetate hydrolase) mouse model.6 Another group corroborated the exclusive capacity of HSC cells to differentiate into hepatocytes using additional functionally rigorous markers that defined the population with higher HSC activity frequency.8 These enriched HSC cells differentiated in vitro into albumin-expressing hepatocyte-like cells with extremely rapid kinetics.9 Although several followed studies have reported hepatocyte differentiation from bone marrow cells,10, 11, 12 all these studies evaluated only the phenotypes of initial population and the final differentiated functioning hepatocytes, irrespective of whether an in vivo or in vitro protocol was used.6, 7, 8, 9, 10, 11, 12 Moreover, these studies did not characterize the sequential differentiation process, including key developmental intermediate cells and did not identify the mode of differentiation, that is, transdifferentiation or cell fusion. Furthermore, subsequent studies had difficulty reproducing these results using the published protocols.2, 5, 17
In this study, we aimed to understand and recapitulate in vivo hepatocyte differentiation using in vitro cultures of immature bone marrow cells using several different additives. We established an efficient in vitro culture protocol that resulted in differentiation of functioning hepatocytes from lineage-negative (Lin−) bone marrow cells. These cells reduced liver damage and were incorporated into the hepatic parenchyma in two independent hepatic injury models. Our simple and effective initial protocol of expanding immature bone marrow cells revealed that Foxa2+ endodermal precursor cells exist in Sca1+ subpopulations of Lin− cells. Also, these endodermal precursor cells followed a sequential developmental pathway that led to functioning hepatocytes through physiologically intermediate endodermal and hepatocyte precursor cells.
Materials and methods
Animals
C57BL/6 (B6) mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). Experiments involving mice were approved by the Institutional Animal Care and Use Committee of Seoul National University (Seoul, Korea; authorization no. SNU05050203).
Bone marrow cells and purification of lineage-negative cells
Bone marrow cells were obtained from the tibia and femur of mice. Lineage-positive (Lin+) cells were depleted by magnetic-activated cell sorting using an APC-conjugated mouse lineage antibody cocktail (BD Pharmingen, San Diego, CA, USA) and anti-APC microbeads (Miltenyi Biotec, Auburn, CA, USA). After magnetic-activated cell sorting purification, the purity of Lin− cells was >95% in all experiments. For in vivo and in vitro donor cell tracking experiments, Lin− cells were labeled with PKH26 (Sigma-Aldrich, St Louis, MO, USA) or Vybrant DiI (Molecular Probes, Eugene, OR, USA) and stained with anti-Sca1 and anti-c-Kit antibodies (BD Pharmingen) and sorted using BD FACSAriaIII (BD Bioscience, San Jose, CA, USA). The purity of each sorted population was >99%.
Preparation of murine serum and liver-conditioned medium
Murine serum (MS) was obtained from untreated adult mice. For preparation of liver-conditioned medium (LCM), mice were killed and livers were cut into ~1 mm3 pieces under a dissection microscope. The tissue blocks were equally seeded in 35-mm-diameter dishes at a density of 70 tissue blocks per dish. When the blocks adhered to the bottom of the dishes, 1.5 ml basic medium was added containing Iscove’s Modified Dulbecco’s Medium (Gibco Life Technologies, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (Gibco Life Technologies), 1 × minimum essential medium (MEM) nonessential amino acids (Gibco Life Technologies), 1 mM L-glutamine (Gibco Life Technologies), 1 × antibiotic-antimycotic (Gibco Life Technologies) and 10 μM 2-mercaptoethanol (Sigma-Aldrich) and the tissues incubated at 37 °C in a 5% CO2 incubator for 48 h. Supernatants were collected and treated with RNase (10 μg ml−1, Intron Biotechnology, Gyeonggi-do, Korea) and DNase (40 μg ml−1, Roche, Indianapolis, IN, USA) at 37 °C for 1 h, and passed through a 0.2 μm filter. The filtrate was designated as LCM and stored in aliquots at –80 °C for future use.
Hepatocyte differentiation in vitro
Lin− cells were seeded onto coverslips coated with type-I-A collagen (100 μg ml−1, Nitta Gelain NA, Osaka, Japan) in a 12-well plate. Cells were cultured in basic medium at 37 °C in a 5% CO2 incubator for 15 days. During the initial 6 days of culture 10% normal MS was added, followed by 10% LCM, 10 ng ml−1 recombinant human hepatocyte growth factor (HGF, R&D Systems, Minneapolis, MN, USA) and 2 μg ml−1 anti-transforming growth factor β blocking antibody for the following 9 days.
Immunofluorescence microscopy
Cells at each differentiation stage were fixed with 4% paraformaldehyde and stained with the appropriate antibody. The following antibodies were used: anti-mouse Foxa2 (Abcam, Cambridge, MA, USA), anti-mouse Gata4 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-mouse Trop2 (R&D Systems), A6 antibody (kindly given by Dr Valentina Factor), anti-mouse albumin (Santa Cruz Biotechnology), Alexa 546-conjugated donkey anti-rabbit IgG (Invitrogen Life Technologies, Carlsbad, CA, USA), Alexa 647-conjugated donkey anti-goat IgG (Invitrogen) and Alexa 488-conjugated donkey anti-mouse IgG (Invitrogen). Image acquisition and processing was performed using a confocal fluorescence microscope (Olympus, Center Valley, PA, USA) and an FV10-ASW 2.0 Viewer (Olympus).
EdU incorporation assay
To identify proliferating cells, the Click-iT EdU Alexa Fluor 647 Imaging Kit (Life Technologies, Carlsbad, CA, USA) was used. EdU (5-ethynyl-2'-deoxyuridine; 5 μM) was added to the culture for 48 h and EdU-positive cells were detected according to the manufacturer’s instructions.
Reverse-transcriptase polymerase chain reaction (RT-PCR)
Total RNA from cultured cells was isolated using TRIzol reagent (Life Technologies). RNA from LCM was isolated using ExoQuick-TCTM exosome precipitation solution (System Biosciences, Mountain View, CA, USA). cDNA was synthesized from 500 ng of total RNA using PrimeScript reverse transcriptase (TAKARA, Shiga, Japan) and subjected to PCR. The following primer pairs were used for PCR: Gapdh, 5′-GTCAGTGGTGGACCTGACCT-3′ and 5′-AGGGGTCTACATGGCAACTG-3′; Alb, 5′-GTGCAAGAACTATGCTGAGG-3′ and 5′-ACTCACTGGGGTCTTCTCAT-3′; Ck18, 5′-GATTGACTGTGGAAGTGGATGC-3′ and 5′-GTTTGCATGGAGTTGCTGGA-3′; Afp, 5′-CACTGCTGCAACTCTTCGTA-3′and 5′-CTTTGGACCCTCTTCTGTGA-3′; Trop2, 5′-GTGTGCTGGTGCGTAAACTC-3′and 5′-ATGGTGGGCTCCTCATAGTG-3′; Dlk1, 5′-TGTCAATGGAGTCTGCAAGG-3′ and 5′-AGGGAGAACCATTGATCACG-3′; Sox9, 5′-TGCAGCACAAGAAAGACCAC-3′ and 5′-CCCTCTCGCTTCAGATGAAC-3′; Tbx, 5′-TTCCCCCTTCGTGTGTTTAG-3′ and 5′-GGACTTCCGTTGTTTCCA-3′; Foxa2, 5′-GACATACCGACGCAGCTACA-3′ and 5′-GGCACTGGAAAGCAGTC-3′; Gata4, 5′-GAGTGTGTCAATTGTGGGGC-3′ and 5′-CTGCTGTGCCCATAGTGAGA-3′; Gata6, 5′-CGGAGGAAAGTACAGAC-3′ and 5′-GAGTAGGGAAAGCGTGCAG-3′; Sox17, 5′-CTCGGGGATGTAAAGGTGAA-3′ and 5′-GCTTCTCTGCCAAGGTCAAC-3′; and Sox7, 5′-GCAGGAAGAAACAAGGCAAG-3′ and 5′-GTGGAGGGGACTAGGTGTGA-3′. PCR products were analyzed by 1.5% agarose gel electrophoresis. Relative levels of mRNA were normalized to the values of Gapdh mRNA for each reaction.
Western blot analysis
Cells were harvested in lysis solution containing 50 mM Tris/HCl (pH 7.6), 1% NP40, 150 mM NaCl, 2 mM EDTA, 100 μM PMSF, a protease inhibitor cocktail (Roche Applied Science), and a phosphatase inhibitor (Sigma-Aldrich). After incubation on ice for 30 min, cellular debris was removed by centrifugation (10 min, 4 °C). Proteins (10 μg) were separated by SDS–polyacrylamide gel electrophoresis and then transferred to a polyvinylidene difluoride membrane. After blocking with 5% skim milk, the membranes were probed with an appropriate antibody. Blots were developed with an enhanced chemiluminescence western blotting detection system (Amersham, GE Healthcare, Buckinghamshire, UK). The following antibodies were used: anti-β-actin (Sigma-Aldrich) and anti-mouse albumin (Santa Cruz Biotechnology).
Hepatic injury models
To induce hepatic injury, 8-week-old female mice were injected intraperitoneally with a single dose of 300 mg kg−1 N-acetyl-p-aminophenol (APAP) or three doses of 10% CCl4 in olive oil (10 μl g−1 body weight) at 3-day intervals. Differentiating hepatocyte-like cells were transferred to mice via intrasplenic injection 8 h after APAP treatment or 24 h after the first CCl4 treatment. Serum was collected to determine serum alanine aminotransferase levels on day 2 after APAP or after the final treatment with CCl4. Mice were killed and the liver was either fixed with 4% paraformaldehyde for histological examination or embedded in optimum cutting temperature (OCT) compound and rapidly frozen and sectioned to trace the transferred cells.
Statistical analysis
All values represent means±s.d. Statistical significance was determined using Student’s t-test. P<0.05 indicated statistical significance.
Results
Hepatocyte differentiation from in vitro culture of mouse Lin– bone marrow cells
We established an efficient in vitro culture model that led to hepatocyte differentiation of a murine Lin− bone marrow population. Among various additives we found that normal untreated adult MS provided the best condition for survival and expansion of Lin− cells during initial 6-day culture. Then culture medium was changed to medium containing LCM and recombinant human HGF, and an anti-transforming growth factor β blocking antibody for a further 9 days to induce hepatocyte differentiation (Figure 1a). Albumin-expressing hepatocyte-like cells with epithelial characteristics were evident in the extended cultures (Figures 1b and c). Treatment with MS without LCM or LCM without initial MS treatment did not lead to differentiation of albumin-expressing cells, if any (Figures 1b and c).
In vitro hepatocyte-like cell differentiation of Lin− bone marrow cells. (a) Murine Lin− bone marrow cells were treated with normal adult mouse serum (MS) for 6 days in basal culture medium and subsequently cultured with LCM, recombinant HGF and anti-transforming growth factor β (anti-TGFβ) antibody for an additional 9 days. (b) Cell morphology was observed on day 15 under a light microscope. (c) Albumin expression (green) on day 15 was analyzed by IF. (d) Albumin (Alb) mRNA expression in LCM and differentiated cells on day 15 in untreated or RNase/DNase-treated LCM. (e) Albumin-expressing cells (green) on day 15 were observed by confocal microscopy; scale bars=50 μm. (f) Albumin staining of cells (green) on the indicated days; scale bars=50 μm. (g, h) Albumin expression levels during the progression of differentiation were analyzed by PCR with reverse transcription (g) and western blot (h), respectively. All data shown are representative of at least three independent experiments.
To rule out the possibility that albumin signals originated from the LCM added during culture rather than from differentiated hepatocyte-like cells, we used LCM pretreated with RNase/DNase. PCR with reverse transcription analysis of albumin expression using RNA extracted from either untreated LCM or RNase/DNase-pretreated LCM was negative (Figure 1d). In addition, albumin expression and albumin-positive hepatocyte-like cell differentiation were similar in the presence of LCM pretreated with RNase/DNase compared with cells with untreated LCM, as determined by PCR with reverse transcription (Figure 1d) and immunofluorescence (IF) microscopy (Figure 1e). These results indicated that murine Lin− bone marrow cells differentiated into albumin-expressing hepatocyte-like cells in sequential in vitro culture with normal MS and LCM. The numbers and percentages of albumin-expressing hepatocyte-like cells, as well as albumin expression levels, increased gradually from culture days 9–15 (Figures 1f–h). Thus, LCM and HGF treatment during the second phase of in vitro culture efficiently induced hepatocyte differentiation of initially expanded immature bone marrow cells.
Characterization of hepatocyte precursor cells following in vitro culture of murine bone marrow cells
During intrauterine development, hepatocytes are differentiated from bipotential precursor cells known as hepatoblasts, which express alpha-fetoprotein (Afp), Dlk1, Hex and Prox1.18 In the adult liver, oval cells characterized as expressing Dlk1 and Trop2 and stained positively with A6 antibody are reportedly involved in certain forms of hepatic regeneration.19 IF microscopic analysis of in vitro differentiating cells revealed that the expression levels of the oval cell markers, Trop2 and A6, were high on culture day 9 and decreased but still present on culture day 12 (Figures 2a and b). PCR with reverse transcription analysis showed that expression of Afp began to appear at day 9 and peaked on days 12 and 15 of culture. Dlk1 expression was highest on culture day 9, that of Sox9 was highest on culture day 7 and decreased thereafter, while Tbx expression also peaked on culture day 7 and was maintained at high levels after culture day 9 (Figure 2c). Thus, hepatocyte-like cells developed in vitro from Lin− bone marrow cells that were differentiated into hepatocyte precursor cells, which share markers of oval cells or hepatoblasts.
Hepatocyte precursor-like cells appeared before hepatocyte differentiation. Anti-Trop2 (green) and A6 (red) antibodies were used to detect hepatocyte precursors in the cells on day 9 (a) and day 12 (b) of differentiation. Nuclei were counterstained with DAPI (blue); scale bars=50 μm. (c) Expression of hepatocyte precursor markers during the progression of differentiation was determined by PCR with reverse transcription. All data shown are representative of at least three independent experiments.
Hepatocyte precursor cells were differentiated through endodermal cell stage in vitro
To evaluate whether hepatic precursor cells developed in vitro via a physiological route that included the endoderm stage,20, 21, 22 we analyzed the expression kinetics of endodermal markers. Foxa2, Gata4, Gata6, Sox7 and Sox17 were first detected on culture day 3, peaked on day 6 and decreased thereafter (Figure 3a). Differentiating endodermal cells co-expressing Foxa2 and Gata4 were identified on culture day 6 by IF microscopy (Figure 3b). These findings indicated that in vitro differentiation of hepatocyte-like cells from Lin− cells recapitulated the natural differentiation process via sequential development through the endodermal and bipotential hepatocyte precursor stages.
Expression of endodermal markers during the early stages of differentiation. (a) Expression of endodermal markers was determined by PCR with reverse transcription on the indicated days. (b) Co-expression of Foxa2 (red) and Gata4 (green) on day 6 of differentiation. Nuclei were counterstained with DAPI (blue); scale bars=50 μm. All the data shown are representative of at least three independent experiments.
Proliferative capacity of differentiating cells in vitro
The proliferative capacities of sequentially differentiating endodermal cells, hepatocyte precursor cells and hepatocyte-like cells in vitro were evaluated using EdU incorporation and a click-chemistry visualization system.23 EdU is a thymidine analog incorporated into DNA during the S-phase of cell proliferation.23 The percentage of EdU+ proliferating cells among total nucleate cells was 21.51±3.70% on culture day 3 and 45.63±4.40% on culture day 6 (Figure 4a). EdU+ cell percentages decreased from culture day 6 under differentiation conditions in the presence of LCM and HGF, as follows: 15.80±6.69% on culture day 9 and 3.01±1.98% on culture day 12 (Figure 4a). These findings revealed that the initial culture conditions (with MS) simultaneously enhanced the proliferative capacity and survival/differentiation of endodermal cells. However, differentiation into hepatocyte precursor cells and hepatocyte-like cells with LCM and HGF compromised the in vitro proliferative capacity of these cells.
Proliferative capacity of progenitor cells. Cells were cultured in the presence of 5 μM EdU for 48 h during differentiation and analyzed on the indicated days. (a) The number of EdU+ cells was expressed as a ratio to the total number nucleated cells. The data are presented as means±s.d. from three independent determinations using samples from three cultures. (b) EdU+ (gray) cells among the Foxa2 (red)- and Sox17 (green)-expressing cells were analyzed at days 3 and 6. Nuclei were counterstained with DAPI (blue). Scale bars=50 μm. (c) The ratio of Foxa2+EdU+ cells to total Foxa2+ cells was calculated using the ImageJ software. Data represent means±s.d. based on three independent determinations using samples from n=3 cell cultures. (d) EdU+ (gray) cells among the A6 (red)- and Trop2 (green)-expressing cells were analyzed at days 9 and 12. Nuclei were counterstained with DAPI (blue); scale bars=50 μm. (e) The ratio of Trop2+EdU+ cells to total Trop2+ cells was calculated. Data represent means±s.d. based on three independent determinations using samples from n=3 cell cultures. Data in B and D are representative of at least three independent experiments.
Next, we analyzed the expression in proliferating cells of molecules specific for different stages of hepatocyte differentiation using combined IF and EdU incorporation assays. On culture days 3 and 6, substantial percentages of endodermal cells co-expressing Foxa2 and Sox17 were EdU+ (Figure 4b). The percentage of Foxa2-expressing cells among total nucleate cells was 40.13±3.28% on day 3 and 46.36±2.38% on day 6 (Figure 4c). The percentage of proliferating cells among Foxa2-expressing cells (Foxa2+EdU+) was 23.05±2.74% on day 3 and 49.41±8.96% on day 6 (Figure 4c). Regarding hepatocyte precursor cell markers, cells co-expressing Trop2 and A6 and EdU+ were identified on days 9 and 12 (Figure 4d). The percentage of Trop2-expressing cells among total nucleated cells was 47.32±4.86% on day 9 and 46.77±5.87% on day 12 (Figure 4e). The percentage of proliferating cells among Trop2-expressing cells (Trop2+EdU+) was 26.52±9.42% on day 9 and 5.16±3.63% on day 12 (Figure 4e). Thus, the initial in vitro culture with MS resulted in extensive proliferation and endodermal differentiation of bone marrow cells, and culture with LCM and HGF induced hepatocyte precursor and hepatocyte-like cell differentiation and reduced their in vitro proliferative capacity.
Differentiating hepatocyte-like cells reduced liver damage and incorporated into the hepatocyte plates in two hepatic injury models
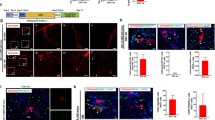
To evaluate the in vivo functional capacity of differentiating hepatocyte-like cells, we utilized two independent hepatic injury models. Acetaminophen (APAP)-induced acute and CCl4-induced subacute liver injury models were generated, and differentiating hepatocyte-like cells were transferred via intrasplenic injection 8 h after APAP treatment or 24 h after the first dose of CCl4 treatment. Serum alanine aminotransferase levels were decreased in the hepatocyte-like cell transfer group 2 days after final treatment with CCl4 compared to the vehicle-treated controls (Figure 5a). To confirm the effective replacement of in vitro differentiated cells into the damaged liver parenchyma, we stained hepatocyte-like cells either with DiI or PKH26 before adoptive transfer. We found that DiI- or PKH26-positive and albumin-expressing hepatocytes were incorporated into the hepatic parenchyma and constituted normal-like hepatocyte plate structures in the cell entry site near portal area in the CCl4- and APAP- induced hepatic injury models (Figures 5b and c).
Engraftment of in vitro differentiated hepatocyte-like cells into the injured livers. (a–c) Lin− cells were differentiated in vitro for 15 days and labeled with Vybrant DiI or PHK26. Labeled cells (2 × 106) were transferred by intrasplenic injection into CCl4- or APAP-injected mice. (a) Serum alanine aminotransferase levels were determined 2 days after the final treatment with CCl4 (n=8 mice) or vehicle (n=6 mice). (b) Liver tissues from CCl4-injected mice were prepared 17 days after cell transfer. Albumin-expressing DiI+ cells were detected by IF microscopy; scale bars=50 μm. Representative photographs of livers are shown (n=6 mice per group). (c) Liver tissues from APAP-injected mice were prepared 7 days after cell transfer. Albumin-expressing PKH26+ cells were detected by IF microscopy; scale bars=50 μm. Representative photographs of livers are shown (n=4 mice per group).
Lin− bone marrow cells contain a Foxa2+ endodermal precursor cell population
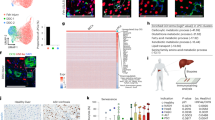
A previous report revealed that HSCs using KTLS markers (c-KithighThyloLin−Sca1+) were the only cell types that could differentiate into hepatocytes in an in vivo transfer model.6 In addition, Fr25Lin− marrow-homing cells had differentiated into multiple cell types, including hepatocytes following in vivo transfer8 and these cells differentiated into hepatocyte-like cells in vitro within 7 days.9 We questioned the initial phenotypes of bone marrow cells that could differentiate into hepatocytes in our system in terms of KLS markers. We stained Lin− cells with DiI and sorted them with >99% purity into four subpopulations based on Sca1 and c-Kit expression (Figure 6a). We cultured the sorted DiI-stained cells with unstained Lin− cells and traced the in vitro fate of DiI+ cells. DiI+Trop2+ hepatocyte precursor development was evident in three of the four subpopulations (Sca1+c-Kit+, Sca1+c-Kit− and Sca1−c-Kit−, but not Sca1−c-Kit+) on culture day 9 (Figures 6b and c). We further questioned whether the endodermal cells were generated in culture or whether they existed in the Lin− cells as endodermal precursor cells by evaluating four highly purified subpopulations with IF staining. We found that the same three of the four subpopulations contained Foxa2+ cells; these Foxa2+ cells were Gata4−/lo and considered to be precursors of Foxa2+Gata4+ endodermal cells (Figures 6d and e). We evaluated the expansion of DiI-stained purified cells during the initial 6-day culture. The initial proliferative capacity was evaluated by counting DiI+ cells on days 0 and 6 of culture, and was highest in the Sca1+c-Kit− population, followed by the Sca1+c-Kit+ population (Figure 6f). These findings suggest that Sca1+ subpopulations contributed to the hepatocyte-differentiating capacity of Lin− cells.
Lin− cells contained Foxa2+Gata4+/low cells. (a) The sorted Lin− cells were analyzed for expression of Sca1 and c-Kit using flow cytometry. (b, c, f) Four subpopulations (c-Kit+Sca1+, c-Kit−Sca1+, c-kit−Sca1− and c-Kit+Sca1−) of Lin− cells were sorted and labeled with Vybrant DiI dye. DiI-labeled cells (6000 cells) and unlabeled Lin− cells (2.5 × 106 cells) were co-cultured and differentiated. (b, c) Cells at day 9 were stained with anti-Trop2 Ab. DiI+ (red) and Trop2+ (green) cells and analyzed by IF microscopy. Nuclei were counterstained with DAPI (blue); scale bars=20 μm. (d, e) The sorted four subpopulations were centrifuged onto glass slides (cytospins) and stained with anti-Foxa2 and anti-Gata4 Ab. Cells expressing Foxa2 (red) or Gata4 (green) were detected by IF microscopy. Nuclei were counterstained with DAPI (blue); scale bar=20 μm. (f) Dil+ cells were counted at 0 and 6 days of culture and the fold changes calculated. Data represent means±s.d. based on three independent determinations using samples from n=3 cell cultures.
Discussion
In this study we established effective in vitro culture conditions for differentiation of functioning hepatocyte-like cells from murine Lin− bone marrow cells. Unlike previous reports that characterized only the initial and final cell populations,6, 8, 9, 10, 12 we systematically demonstrated that hepatocyte differentiation followed physiological development pathways through sequential intermediate stages. The critical difference between our current study and previous reports lies in our initial simple and efficient endodermal cell expansion protocol. Initial culture with normal untreated adult MS-induced endodermal cell expansion and differentiation, and albumin-expressing hepatocyte-like cell differentiation through precursor cell stages occurred during the later phase protocol with LCM and HGF. The presence of normal MS enhanced endodermal proliferation and differentiation and LCM and HGF induced differentiation but compromised proliferation.
This simple and efficient procedure enabled us to identify endodermal precursor cells in the Sca1+ subpopulations of Lin− cells and determine that these cells followed sequential developmental pathways through physiological intermediate cells. Previous protocols for hepatocyte differentiation from bone marrow cells unanimously used hepatocyte-conditioned medium or HGF from the beginning, which prevented expansion or evaluation of the putative endodermal precursor cells.9, 10 Transfer of bone marrow cells into mice cannot be used to trace cellular differentiation.6, 8 Thus, most previous studies concluded that hepatocyte differentiation occurred either through direct transdifferentiation or cell fusion.10, 24 Therefore, earlier KTLS or Fr25Lin− populations may also contain endodermal precursor population,6, 8 while hepatocyte differentiation from Fr25Lin− cells in vitro within 7 days9 closely matched the in vitro differentiation kinetics in this study. Our endodermal precursor cell populations were related to bone-marrow-oval-cell-marker-positive cell populations or bone-marrow-albumin-positive cell populations.25, 26 We concluded that hepatocyte differentiation from bone marrow cells was not mediated by transdifferentiation or cell fusion but through physiological sequential differentiation from existing endodermal precursor cells, which might also harbor hematopoietic differentiation potential.
By IF analysis, we found that Foxa2+ endodermal precursor cells were present in adult murine bone marrow cells, and our in vitro protocol yielded efficient initial expansion of these cells.27 Day 6 endodermal cells also had the potential to differentiate into Pdx1+ pancreatic precursor cells in vitro (data not shown). Unlike an in vivo system, our in vitro system evaluated individual cell fate, demonstrated hepatocyte differentiation directly from bone marrow cells, and eliminated cell fusion as an underlying mechanism.
Bone marrow is the largest reservoir of multipotent stem cells, particularly of the two major stem cell populations, HSCs and mesenchymal stem cells (MSCs);3, 17 however, it remains unclear which bone marrow stem cell population is most effective in the regeneration of injured liver tissues.16 A pioneering study suggested that HSCs (c-KithiThyloLin−Sca1+) could give rise to hepatocytes and rescue FAH−/− mice,6 although subsequent studies had questioned these findings.5 Thus, MSCs-derived from bone marrow and other tissues were focused upon and many studies used MSCs as starting materials for hepatocyte differentiation.28, 29 Under our culture conditions, the differentiating cells did not assume MSC characteristics in vitro and featured endodermal epithelial-like phenotypes early in culture with normal untreated MS, which ruled out the possibility that hepatocyte cell differentiation in our study occurred through MSCs.
The phenotypic characteristics of the initial Lin− bone marrow cells used in our study require further discussion. Previous reports confined hepatocyte-differentiation capacity to HSCs, as defined by KTLS markers or more rigorously using Fr25Lin− 48-h marrow-homing cells.6, 8 Extended differentiation of these HSCs into epithelial cells of both ectodermal and endodermal origin was also established.8 In our study, we evaluated the surface phenotype using KLS markers and found that Trop2+ hepatocyte precursor cell differentiation was not confined to the KLS population but also occurred in the Sca1+c-Kit−Lin− subpopulation, which also contained substantial numbers of precursor cells with capacity for hepatocyte differentiation. The Sca1+Lin− population evaluated in our study was comparable to previous reports of enriched hepatocyte-like cell differentiation potential among the Sca1+c-Kit− population.10 Since previous reports revealed that bone marrow cells generating hepatocytes also produced blood cells,6, 8 we need to evaluate whether our endodermal precursor cells in the Sca1+Lin− populations could differentiate into blood cells. Furthermore, it is important to assess whether our initial Sca1+Lin− populations could differentiate into other cell types, such as epithelial cells of ectoderm origin or mesodermal cells.
These data suggest that murine bone marrow cells do not transdifferentiate into cell types of diverse lineages, but inherently contain more diverse and primitive stem cell populations that could develop into conventional three germ layer components depending on their in vitro or in vivo microenvironment.30 Our protocol for differentiation of functioning hepatocytes from bone marrow cells is unusually simple and efficient. Moreover, similar to pluripotent stem cell differentiation protocols, we successfully reiterated the various normal-like sequential developmental stages, which guarantee more physiologically relevant cells.5
Clinical studies have reported that mobilization of bone-marrow-derived CD34+ cells into alcoholic liver cirrhosis patients led to clinical and biochemical improvement.31, 32 Human transplant recipient studies have reported that hepatocytes have been derived from donor bone marrow cells.33, 34 Endodermal stem cell lines from human embryonic stem cells were characterized and could expand endodermal stem cells >1016-fold relatively easily.35 These reports suggest that equivalent endodermal precursor populations exist in human bone marrow, allowing for efficient in vitro expansion capacity. Our finding of endodermal precursor cells in murine bone marrow and development of efficient expansion protocols could justify the use of these cells to treat liver disease in the near future and provide an important breakthrough in liver regenerative medicine.
References
Huebert RC, Rakela J . Cellular therapy for liver disease. Mayo Clin Proc 2014; 89: 414–424.
Sancho-Bru P, Najimi M, Caruso M, Pauwelyn K, Cantz T, Forbes S et al. Stem and progenitor cells for liver repopulation: can we standardise the process from bench to bedside? Gut 2009; 58: 594–603.
Alqahtani SA . Update in liver transplantation. Curr Opin Gastroenterol 2012; 28: 230–238.
Ben-David U, Benvenisty N . The tumorigenicity of human embryonic and induced pluripotent stem cells. Nat Rev Cancer 2011; 11: 268–277.
Chistiakov DA . Liver regenerative medicine: advances and challenges. Cells Tissues Organs 2012; 196: 291–312.
Lagasse E, Connors H, Al-Dhalimy M, Reitsma M, Dohse M, Osborne L et al. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med 2000; 6: 1229–1234.
Theise ND, Badve S, Saxena R, Henegariu O, Sell S, Crawford JM et al. Derivation of hepatocytes from bone marrow cells in mice after radiation-induced myeloablation. Hepatology 2000; 31: 235–240.
Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R et al. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell 2001; 105: 369–377.
Jang YY, Collector MI, Baylin SB, Diehl AM, Sharkis SJ . Hematopoietic stem cells convert into liver cells within days without fusion. Nat Cell Biol 2004; 6: 532–539.
Yamada Y, Nishimoto E, Mitsuya H, Yonemura Y . In vitro transdifferentiation of adult bone marrow Sca-1+ cKit- cells cocultured with fetal liver cells into hepatic-like cells without fusion. Exp Hematol 2006; 34: 97–106.
Okumoto K, Saito T, Haga H, Hattori E, Ishii R, Karasawa T et al. Characteristics of rat bone marrow cells differentiated into a liver cell lineage and dynamics of the transplanted cells in the injured liver. J Gastroenterol 2006; 41: 62–69.
Khurana S, Mukhopadhyay A . In vitro transdifferentiation of adult hematopoietic stem cells: an alternative source of engraftable hepatocytes. J Hepatol 2008; 49: 998–1007.
Schwartz RE, Reyes M, Koodie L, Jiang Y, Blackstad M, Lund T et al. Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. J Clin Invest 2002; 109: 1291–1302.
Lee KD, Kuo TK, Whang-Peng J, Chung YF, Lin CT, Chou SH et al. In vitro hepatic differentiation of human mesenchymal stem cells. Hepatology 2004; 40: 1275–1284.
Chen Y, Dong XJ, Zhang GR, Shao JZ, Xiang LX . In vitro differentiation of mouse bone marrow stromal stem cells into hepatocytes induced by conditioned culture medium of hepatocytes. J Cell Biochem 2007; 102: 52–63.
Ochiya T, Yamamoto Y, Banas A . Commitment of stem cells into functional hepatocytes. Differentiation 2010; 79: 65–73.
Wu XB, Tao R . Hepatocyte differentiation of mesenchymal stem cells. Hepatobiliary Pancreat Dis Int 2012; 11: 360–371.
Tanaka M, Itoh T, Tanimizu N, Miyajima A . Liver stem/progenitor cells: their characteristics and regulatory mechanisms. J Biochem 2011; 149: 231–239.
Fausto N, Campbell JS . The role of hepatocytes and oval cells in liver regeneration and repopulation. Mech Dev 2003; 120: 117–130.
Zhao R, Duncan SA . Embryonic development of the liver. Hepatology 2005; 41: 956–967.
Zaret KS, Grompe M . Generation and regeneration of cells of the liver and pancreas. Science 2008; 322: 1490–1494.
Zaret KS . Genetic programming of liver and pancreas progenitors: lessons for stem-cell differentiation. Nat Rev Genet 2008; 9: 329–340.
Buck SB, Bradford J, Gee KR, Agnew BJ, Clarke ST, Salic A . Detection of S-phase cell cycle progression using 5-ethynyl-2'-deoxyuridine incorporation with click chemistry, an alternative to using 5-bromo-2'-deoxyuridine antibodies. Biotechniques 2008; 44: 927–929.
Terada N, Hamazaki T, Oka M, Hoki M, Mastalerz DM, Nakano Y et al. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature 2002; 416: 542–545.
Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N et al. Bone marrow as a potential source of hepatic oval cells. Science 1999; 284: 1168–1170.
Oh SH, Witek RP, Bae SH, Zheng D, Jung Y, Piscaglia AC et al. Bone marrow-derived hepatic oval cells differentiate into hepatocytes in 2-acetylaminofluorene/partial hepatectomy-induced liver regeneration. Gastroenterology 2007; 132: 1077–1087.
Burtscher I, Lickert H . Foxa2 regulates polarity and epithelialization in the endoderm germ layer of the mouse embryo. Development 2009; 136: 1029–1038.
Ishikawa T, Banas A, Hagiwara K, Iwaguro H, Ochiya T . Stem cells for hepatic regeneration: the role of adipose tissue derived mesenchymal stem cells. Curr Stem Cell Res Ther 2010; 5: 182–189.
Banas A, Teratani T, Yamamoto Y, Tokuhara M, Takeshita F, Quinn G et al. Adipose tissue-derived mesenchymal stem cells as a source of human hepatocytes. Hepatology 2007; 46: 219–228.
Binas B, Verfaillie CM . Concise review: Bone marrow meets blastocyst: lessons from an unlikely encounter. Stem Cells 2013; 31: 620–626.
Pai M, Zacharoulis D, Milicevic MN, Helmy S, Jiao LR, Levicar N et al. Autologous infusion of expanded mobilized adult bone marrow-derived CD34+ cells into patients with alcoholic liver cirrhosis. Am J Gastroenterol 2008; 103: 1952–1958.
Lyra AC, Soares MB, da Silva LF, Fortes MF, Silva AG, Mota AC et al. Feasibility and safety of autologous bone marrow mononuclear cell transplantation in patients with advanced chronic liver disease. World J Gastroenterol 2007; 13: 1067–1073.
Theise ND, Nimmakayalu M, Gardner R, Illei PB, Morgan G, Teperman L et al. Liver from bone marrow in humans. Hepatology 2000; 32: 11–16.
Alison MR, Poulsom R, Jeffery R, Dhillon AP, Quaglia A, Jacob J et al. Hepatocytes from non-hepatic adult stem cells. Nature 2000; 406: 257.
Cheng X, Ying L, Lu L, Galvão AM, Mills JA, Lin HC et al. Self-renewing endodermal progenitor lines generated from human pluripotent stem cells. Cell Stem Cell 2012; 1: 371–384.
Acknowledgements
This work was supported by a grant from the Korea Healthcare Technology R & D Project, Ministry of Health & Welfare, Republic of Korea (Grant no. A120273).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Oh, K., Shon, S., Seo, M. et al. Murine Sca1+Lin− bone marrow contains an endodermal precursor population that differentiates into hepatocytes. Exp Mol Med 47, e187 (2015). https://doi.org/10.1038/emm.2015.64
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/emm.2015.64