Abstract
Mutations identified in the fibrillin-1 (FBN1) gene have been associated with Marfan syndrome (MFS). Molecular analysis of the gene is classically performed in probands with MFS to offer diagnosis for at-risk relatives and in children highly suspected of MFS. However, FBN1 gene mutations are found in an ill-defined group of diseases termed ‘type I fibrillinopathies’, which are associated with an increased risk of aortic dilatation and dissection. Thus, there is growing awareness of the need to identify these non-MFS probands, for which FBN1 gene screening should be performed. To answer this need we compiled the molecular data obtained from the screening of the FBN1 gene in 586 probands, which had been addressed to our laboratory for molecular diagnosis. In this group, the efficacy of FBN1 gene screening was high in classical MFS probands (72.5%,), low (58%) in those referred for incomplete MFS and only slight (14.3%) for patients referred as possible MFS. Using recursive partitioning, we found that the best predictor of the identification of a mutation in the FBN1 gene was the presence of features in at least three organ systems, combining one major, and various minor criteria. We also show that our original recommendation of two systems involved with at least one with major criterion represents the minimal criteria because in probands not meeting these criteria, the yield of mutation identification drastically falls. This recommendation should help clinicians and biologists in identifying probands with a high probability of carrying a FBN1 gene mutation, and thus optimize biological resources.
Similar content being viewed by others
Introduction
Marfan syndrome (MFS, OMIM#154700) is an inherited autosomal dominant disorder of connective tissue with an estimated incidence of 1/5000 live births with more than 25% sporadic cases. MFS is characterized by a broad range of clinical manifestations involving the skeletal, ocular, cardiovascular, integument, pulmonary, and central nervous systems with great phenotypic variability.1, 2 Cardiovascular involvement in the form of aortic aneurysm or dissecting aorta is the most serious life-threatening aspect of the syndrome and can be prevented by timely cardiovascular surgery. Mutations in the gene encoding fibrillin-1 (FBN1, OMIM#134797) cause MFS, as well as other related disorders of connective tissue grouped under the generic term of type-1 fibrillinopathies.3 We have shown that FBN1 mutations are associated with an increased risk of aortic dilatation and dissection, whatever may be the clinical presentation.4
The coding sequence of the FBN1 gene is spread over 65 coding exons, and contains 43 calcium binding (cb) epidermal growth factor-like (EGF) modules.5, 6 Private mutations have been identified over the entire length of the gene with no phenotypic association or apparent clustering in any specific region, with the exception of the severe neonatal form of the syndrome associated with mutations located between exons 24 and 32.7, 8, 9, 10, 11 The detection of a mutation, thus long, difficult and costly, cannot be offered to all suspected Marfan patients even in countries, in which state funding is available for wide molecular diagnosis. Therefore, we recommended earlier11 that FBN1 molecular analysis be performed in newly suspected MFS when two systems are involved with at least one major diagnostic criterion (as described in the Ghent nosology). In patients found to harbor a mutation, this recommendation allows for diagnosis of MFS. However, FBN1 molecular analysis must also be performed in the ill-defined group of type I fibrillinopathies, as they carry an increased risk of cardiovascular disease.11 Therefore, the question arises of when to perform FBN1 gene mutation screening in patients with incomplete phenotypes. To answer this question, we first compiled the molecular data obtained from the screening of the FBN1 gene in 586 probands, which had been addressed to our laboratory for molecular diagnosis. We then performed recursive partitioning on the molecular data obtained for probands, in which the requirements of the Ghent nosology for diagnosis of MFS were not met. We built a regression tree to identify combinations of clinical features related to mutation rate detection. We found that in patients with full screening of the Ghent criteria, but dural ectasia (as this feature is not systematically looked for in clinical practice)11 the best predictor of the identification of a mutation in the FBN1 gene was the presence of an ectopia lentis (EL) or features in at least three organ systems, combining one major and various minor criteria. We also found that our original recommendation of two systems involved at least one with major criterion, represents the minimal criteria because in probands not meeting these criteria, the yield of mutation identification drastically falls.
Materials and methods
Patients
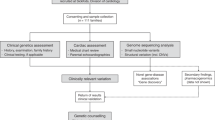
Blood samples were obtained for probands originating from all over the national territory and referred for molecular diagnosis to our laboratory (the first French national reference laboratory for MFS) between 1994 and 2006. Informed consent was provided for all patients in agreement with the French bioethic laws. Since 1996, the majority of patients were referred by the Multidisciplinary Marfan Clinic of our University Hospital. There, patients are evaluated by geneticists, rheumatologists or pediatricians (depending on their age), cardiologists, and ophthalmologists. Systematic slit-lamp examination, cardiac ultrasonography, and radiological investigations are also performed. Dural ectasia is not systematically searched by magnetic resonance (MR) or computed tomography (CT). For samples referred from other centers, the clinical data of patients were routinely collected before mutation screening, but complete data were not always available. Patients with missing data (except neurological screening) were not classified and were not included in the statistical analyses. All samples referred were not screened. Screening exclusion criteria evolved through the years due to a revision of the international diagnostic criteria for MFS,12, 13 but generally probands with an isolated system involvement were not screened (except for familial cases of EL or aortic aneurysm/dissection). For the purpose of this study, all clinical data were reassessed by one physician (C.S.), using the Ghent criteria12 to ensure homogeneity of clinical data across centers. In current practice, only readily available clinical data are used for initial evaluation, and imaging in search of dural ectasia is rarely performed.11 In keeping with this, only 186 of the 586 probands had an investigation of the lumbar spine by CT or MR imaging. Therefore, we relied mainly on the involvement of the skeletal, ocular, and cardiovascular systems to classify them. Furthermore, the probands tested were subdivided into neonatal MFS (defined by severe valvular involvement and clinical features of MFS before 4 weeks of age), probable MFS (defined by incomplete Ghent criteria in childhood, that is, <18 years), incomplete Marfan (patients with incomplete Ghent criteria in adulthood, but with at least one system involved with a major criterion and another system with a minor criterion), thoracic aortic aneurysm or dissection (TAAD defined as major aortic involvement without any other system involved), EL, or skeletal involvement only, non-MFS (defined by only minor involvement in one or more systems), and classical MFS (defined by positive Ghent criteria, whatever may be the age).
Finally, 18 probands presenting with phenotypes overlapping MFS were also investigated: Weill-Marchesani syndrome (WMS), Lujan Fryns syndrome, Shprintzen Goldberg syndrome (SGS), Loeys-Dietz syndrome (LDS), Furlong syndrome [(FS) now integrated in the clinical spectrum of LDS type 1A (OMIM#609192)], or Ehlers-Danlos syndrome (EDS) vascular type. All these disorders were diagnosed using recognized criteria.13, 14, 15, 16, 17, 18
DNA amplification and mutation detection
Genomic DNA was isolated from peripheral blood leukocytes by standard procedures. Initially, all the 65 coding FBN1 exons and their splice junctions were amplified using the primers and conditions described by Nijbroek et al8 with the exception of eight newly designed primers for exon 1 (Ex1F: 5′-GAC GGG CGG CGG GAT AGC- 3′; Ex1B: 5′- TGG ATC TTG AAA CTT GGG-3′), exon 5 (Ex5F: 5′-TTT ATT GTT GTC CTT CCA GAG G-3′; Ex5B: 5′-GCC ATG CAG ACC CAA TGT C-3′), exon 47 (Ex47F: 5′-TAT TAA AGG AAT TGT TGG GG-3′; Ex47B: 5′-TTC CAG GTC TTT CTA AGT CC-3′), and exon 49 (Ex49F: 5′-TGA TGA TGT CTC CAT CGT GT-3′; Ex49B: 5′-TGC AGC ATT GAA AGC CCA AA-3′). Subsequently, primers specifically designed to amplify all regions at the same annealing temperature were used (Dr P Khau Van Kien private communication). All PCRs were carried out in the GenAmp PCR System 9700 (Applied Biosystem, Warrington, Cheshire, UK) with reagent master mix (AB gene). Bidirectional sequencing was performed. Altered exons were resequenced on new DNA dilutions prepared from stock DNA (Big Dye terminators kit, ABI 3100 Genetic Analyzer, Applied Biosystems). When the mutation altered the regional restriction map, the presence of the mutation was also checked by PCR/digestion using the appropriate restriction enzyme.
Prediction of the functional effect of amino acid substitution
To assess the deleterious effect of identified sequence variants, we used various algorithms (Polymorphism Phenotyping (PolyPhen); Sorting Intolerant From Tolerant (SIFT), Biochemical values and BLOSUM 62), which were developed to predict whether or not a nucleotide variation is likely to affect protein function. All, except PolyPhen, are implemented in the new version of the UMD-LSDB software in the ‘UMD-Predictor’ tool, kindly provided by Dr C Béroud (manuscript submitted).
Statistical analysis
Qualitative data were compared by the Pearson χ2 test, or Fisher’s exact test for small samples. A P-value of <0.05 was considered significant. A regression tree was built through recursive partitioning to identify combinations of clinical features related to mutation detection rate. The algorithm chooses the split on a variable that partitions the data set into two parts such that it minimizes the sum of the squared deviations from the mean in the separate parts. This splitting or partitioning is then applied to each of the new branches. The process continues until each node reaches a user-specified minimum node size and becomes a terminal node. Stopping rule was a minimum node size of ten. Trees were cross-validated to prevent overfitting. Statistical analyses were performed using SPSS software version 12.0 (Chicago, Ill) and R language (R is a language and environment for statistical computing. It was developed by R Development Core Team (2005); R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL: http://www.R-project.org.).
Results
Mutation analysis
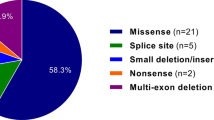
In our set of 586 patients screened by direct sequencing, 354 mutations in 345 probands were identified (success rate of 58.9%). It should be noted that nine patients presented on the same allele, two mutational events predicted as pathogenic. For all probands, the 65 coding exons of the FBN1 gene were studied. Molecular data of each identified mutation are summarized in Supplementary Tables 1–6. Over 200 unrelated French non-MFS patients referred to the laboratory for diagnosis were screened to exclude polymorphisms. In all cases for which family DNA samples were available, complete cosegregation of the mutational event with the clinically affected status was observed. Among the 354 mutations detailed in this report, two mutations detected in this cohort have been described elsewhere (c.2954G>A and c.3965-2A>G (c.IVS31-2A>G));19, 20 and 87 mutations have been made available to the community through the UMD-FBN1 website since 2003.21, 22 Mutations are distributed as follows: 196 missense mutations, 48 alterations affecting conserved splice sites, 55 nonsense mutations, 14 insertions/duplications, and 41 deletions. Among the mutations found, 188 (53.1%) were familial.
Recurrent mutations
All mutations identified in the French probands are newly described mutations except 97 different mutations, which were earlier found in non-French probands (Supplementary Table 1). Among these 97 mutations, 18 were found in more than one French proband. Haplotype analysis, when possible, showed that the mutations were carried on different chromosomal backgrounds or genotyping of the probands’ parents showed a de novo molecular event, thus excluding the existence of a French founder mutation. The most frequent mutation reported in the FBN1 database was one of the first recurrent mutations published, c.5788+5G>A (c.IVS46+5G>A)8, 23 reported 18 times. This mutation was found only in two French probands. Conversely, mutation c.7754T>C initially described by Liu et al24 was found in five unrelated French families, but only reported 10 times in the FBN1 database.
Double mutants
Nine double mutants have been characterized (c.3299G>T;8176C>T), (c.4143G>C;c.3838+1G>C), (c.442C>T;c.1844delA), (c.308dup;c.1957_1958dup), (6388G>A;8176C>T), (c.1416C>A;c.8176C>T), (c.986T>C;c.6832C>T), (c.4057G>A;c.2215T>C), and (c.3909delT;c.3188C>G). For all these probands, parent samples were studied and showed that both mutations were carried on the same allele. Only one case with two mutations has been reported earlier: but for a compound heterozygote (c.3212T>G;c.3219A>T).25
Deletions
Forty-one mutations are deletions (suspected deletion mechanism reported in Supplementary Table 2). Thirty-nine result in a premature termination codon. Two mutations are in-frame: one deletes a cysteine implicated in a disulfide bond, predicting an incorrect folding of the monomer (c.4349_4351delGCT). The second one deletes two amino acids Pro1352 and Gly1353. The Gly1353 is a highly conserved amino acid in cbEGF-like modules. The c.2420delA mutation concerns the first nucleotide of exon 20. However, the consensus splice site is very likely not inactivated by the deletion, as the consensus acceptor splice site value is not modified. The mutational mechanism is then likely to be a frameshift with the appearance of a premature termination codon.
Insertions and duplications
Fourteen mutations were insertions (suspected insertion mechanism reported in Supplementary Table 3). Twelve result in a premature termination codon. Two mutations are in-frame and add a cysteine, predicting an incorrect folding of the monomer.
Splice site mutations
Forty-eight mutations were splice mutations that are predicted to cause abnormal splicing patterns by the use of the nearest and strongest consensus splice site. Thirty-three mutations are located in the intron at the canonical splice sequences. Thirteen of them inactivate an acceptor splice site and twenty a donor splice site. Thirteen mutations are located in exonic sequences of the canonical splice sequences in the last nucleotide of the affected exon (Supplementary Table 4). Two mutations create cryptic acceptor splice sites c.5423-28delCCT (c.IVS43-28delCCT) and c.6997+17delC (c.IVS56+17delC). It is interesting to note that three mutations were located in exonic consensus splice site sequences, but are not likely to modify the used splice site: c.442C>T implicating the last nucleotide of exon 4 (consensus donor splice site value not modified), c.3209A>G mutation implicating the first nucleotide of exon 26 (consensus acceptor splice site value increased), and c.6740A>G mutation implicating the first nucleotide of exon 55 (consensus acceptor splice site value increased). The mutational mechanism in these cases is then most likely an amino acid substitution and these mutations are described in Supplementary Table 6.
Nonsense and missense mutations
Fifty-five nonsense mutations have been identified (Supplementary Table 5). Eight are ocher mutations (TAA), 13 are amber mutations (TAG), and 34 are opal mutations (TGA). One hundred and ninety-six missense mutations have been characterized (Supplementary Table 6). Neutral variants are differentiated from pathogenic nucleotide substitutions by the use of a new tool implemented in the UMD-database (manuscript submitted). This tool takes into account the location of the variant at the protein level, that is, in which domain and whether it is involved in a highly conserved domain. The new algorithm implemented in the UMD-database scored all the mutations we report as pathogenic.
Classification according to predicted mutation consequences
One hundred and fifty-four mutations (representing 42.8% of mutations) are predicted to result in shortened fibrillin-1 molecules. These mutations are distributed as follows: 55 nonsense, 48 splicing errors, 12 frameshift insertions/duplications, and 39 frameshift deletions. Mutations predicting an incorrect folding of the monomer concern 119 mutations: (1) substitution of a cysteine implicated in a disulfide bond is reported in 93 cases; (2) on the other hand, 22 mutations create a new cysteine potentially disturbing the correct cysteine pairing; and (3) four substitutions of a glycine implicated in correct domain–domain packing are reported.26 In the other mutations, 42 mutations concern amino acids known to be implicated in Ca2+ binding.27 Fourteen mutations are located at amino acids highly conserved among species.
Clinical evaluation in our population
The clinical spectrum before FBN1 testing and according to data referred by physicians was the following: 21 cases of neonatal MFS, 21 probable MFS (children), 105 incomplete MFS (adult) (20% of adults), 266 cases of classical MFS (50.5% of adults), 21 non-MFS (4%), and six TAAD, eight EL, one isolated skeletal involvement only. For 119 patients, investigation of one or more systems (other than dural ectasia) was not available and therefore we did not include them in one of the earlier clinical groups and in the statistical analysis. Finally, three Lujan Fryns, six SGS, three WMS, one Furlong syndrome, two EDS vascular types (with a COL3A1-negative gene screening), and three LDS were also studied. These 18 probands were not included in the statistical analysis.
Efficacy of mutation screening
The overall mutation detection rate was 193/266 (72.5%) in patients clinically referred to the laboratory as classical MFS and 61/105 (58%) in those referred as incomplete MFS. Conversely, a very low mutation yield 3/21 (14.3%) was observed for patients referred as possible MFS, but with no major diagnostic criterion, in an organ system. These patients constituted our non-MFS group. The number of mutations detected depending on the nature of an isolated affected system and the involvement of various system combinations was investigated (Table 1).
Among the incomplete MFS patients in whom the FBN1 gene had been screened, we looked for an association between combinations of system involvement and mutation identification rates. For this purpose we performed recursive partitioning, which produced the regression tree shown in Figure 1. Only probands with available (positive or negative) data for all systems (but neurological) were used. This analysis showed that among patients with incomplete MFS, the most important criteria for mutation detection were the number of systems involved and the presence of EL.
Mutation in other diseases
FBN1 gene mutations were found in a few probands carrying overlapping diseases: c.3058A>G in a Lujan Fryns patient, c.2088C>A and c.4270C>G in two SGS patients, c.3761G>A in the only FS-LDS1A patient screened and c.640G>A was detected in one WMS patient. Finally, no mutation was found in the other LDS patients and in EDS patients.
Discussion
This study represents the largest report of FBN1 mutations from a single laboratory and shows that France is one of the leading countries for molecular diagnosis of type 1 fibrillinopathies. In France, until recently, only our laboratory was implicated in FBN1 mutation screening. We chose to apply systematic sequencing of the 65 exons of the FBN1 gene for detection and identification of mutations. This strategy was performed on a series of 586 probands that enabled us to identify 354 mutations in 345 probands.
French mutations compared with earlier reported mutations
The large set of molecular data that we report generally supports the observations made by various teams on much smaller data sets (Supplementary Table 7). The percentage of missense, splice sites, nonsense mutations, insertions/duplications, and deletions are roughly comparable with earlier reported mutations.11, 28, 29 A slight decrease in deletion mutations to the advantage of point mutations, and particularly nonsense mutations, is observed. These mutations are, as earlier described mutations, spread along the FBN1 gene without specific clustering, sign of a mutation hotspot (Supplementary Table 7). An increased number of mutations is found for exons: 2, 15, 22, 27, 46, 55, and 62 and, conversely, an apparent lack of mutations is found in exons 7, 41, and 65. Of all the FBN1 mutations described today (more than 1750, data not published), the great majority (79.1%) is from the European laboratories and especially Western Europe (France, UK, Germany, Italy, Belgium, Norway, and Netherland in order of importance, according to UMD-FBN1). These higher number of mutations found in Europe is related to the fact that FBN1 mutation screening is no longer performed on a regular basis in academic laboratories elsewhere. It is interesting to note that this report is the first to describe several double mutants. In effect, nine probands carried two mutations on the same allele. The lack of comparable information in reports from other teams could be explained either by incomplete screening of the gene when a first mutation is found, or that other teams have in fact only reported the most probably deleterious event.
In our study, we found a significant difference in the yield of FBN1 mutation identification between patients fulfilling and not fulfilling Ghent diagnostic criteria (72.5 vs 58% P=0.0001). This is in agreement with earlier reports that are summarized along with ours in Table 2. More mutations were found in our study ‘incomplete MFS group’ (58%) than in other studies. This may be explained by the fact that our incomplete MFS population is not comparable with those reported by other teams.28, 29, 30, 31 The incomplete MFS groups in the latter are in fact a combination of our incomplete MFS and our non-MFS groups. Furthermore, it should be noted that in all these published studies, the authors report some patients in whom the status of the dura is unknown, thus comparable with our report.4, 28, 29, 30, 31 Taken together results from all studies confirm our first published recommendation:4 that FBN1 gene screening be performed in newly suspected MFS when two systems are involved with at least one major diagnostic criterion.
Identification of indicators for better mutation detection rates
To understand the correlation between organ system involvement and mutation finding, we checked the contribution of each system alone or in various combinations. Taken singly, each organ system, even with a major diagnostic criterion, was not by itself a good predictor of mutation identification (Table 1). However, major ocular involvement in various combinations was always associated with an elevated yield in mutation identification. In the case of major cardiac involvement, an interesting trend was found: mutation detection rate increased by steps for each added minor system involvement. Recursive partition allowed us to assess the relative importance for mutation identification of the type of system involvement and the number of systems involved with a minor diagnostic criterion. This data-mining technique naturally takes into account interaction between variables and produces easy-to-use regression trees. Among the different variables tested, we found that the most significant was the number of organ systems involved. Thus in our study, the best predictor of the identification of a mutation in the FBN1 gene was the presence of features in a wide number of systems. This result further emphasizes the importance of an exhaustive clinical investigation to evaluate the pertinence of performing the molecular analysis. The second most significant variable found in our study was EL. Therefore, the second best predictor of identification of a mutation in the FBN1 gene was the combination of EL and involvement of at least another system. The importance of EL with respect to probability of mutation identification has already been noted.32 However, our study shows that this major diagnostic criterion alone is insufficient.
Recommendations in choice of patients to be screened
Laboratories offering molecular diagnosis for MFS and overlapping disorders must meet a challenge to provide diagnosis in the most relevant clinical situations, while managing costs. Since the identification of the first mutations in the FBN1 gene in MFS patients, laboratories have been flooded with prescriptions for molecular screening of the gene in probands distributed throughout a wide clinical spectrum ranging from neonatal MFS to isolated skeletal overgrowth. Rapidly, most diagnostic laboratories generally performed FBN1 gene screening in three circumstances: first, in probands meeting the clinical criteria for MFS to offer diagnosis for at-risk relatives; second, in children highly suspected of MFS, but failing to meet the diagnostic criteria, as features of the disease appear over time (from childhood to early adult); and third when prenatal diagnosis is considered for an at-risk couple. In the first two circumstances, molecular diagnosis enables proper and early identification of affected patients who need adequate follow-up and treatment to prevent the life-threatening complications of MFS. However, these circumstances exclude a large body of probands with an increased risk of aortic dilatation and dissection because they carry an FBN1 mutation. Therefore, we not only confirm that FBN1 gene screening should be performed in newly suspected MFS when two systems are involved with at least one major diagnostic criterion, but we also show that within this context, the yield of mutation identification will be the highest in probands presenting with EL and involvement of at least another system.
Finally, our data also show that our original recommendation (two systems involved with at least one with major criterion)4 represent the minimal criteria, because in probands not meeting these criteria, the yield of mutation identification drastically falls.
References
Ammash NM, Sundt TM, Connolly HM : Marfan syndrome-diagnosis and management. Curr Probl Cardiol 2008; 33: 7–39.
Judge DP, Dietz HC : Marfan's syndrome. Lancet 2005; 366: 1965–1976.
Boileau C, Jondeau G, Mizuguchi T, Matsumoto N : Molecular genetics of Marfan syndrome. Curr Opin Cardiol 2005; 20: 194–200.
Faivre L, Collod-Beroud G, Loeys BL et al: Contribution of molecular analyses in diagnosing Marfan syndrome and type I fibrillinopathies: an international study of 1009 probands. J Med Genet 2008; 45: 384–390.
Corson GM, Chalberg SC, Dietz HC, Charbonneau NL, Sakai LY : Fibrillin binds calcium and is coded by cDNAs that reveal a multidomain structure and alternatively spliced exons at the 5′ end. Genomics 1993; 17: 476–484.
Pereira L, D’Alessio M, Ramirez F et al: Genomic organization of the sequence coding for fibrillin, the defective gene product in Marfan syndrome. Hum Mol Genet 1993; 2: 1762.
Kainulainen K, Karttunen L, Puhakka L, Sakai L, Peltonen L : Mutations in the fibrillin gene responsible for dominant ectopia lentis and neonatal Marfan syndrome. Nat Genet 1994; 6: 64–69.
Nijbroek G, Sood S, McIntosh I et al: Fifteen novel FBN1 mutations causing Marfan syndrome detected by heteroduplex analysis of genomic amplicons. Am J Hum Genet 1995; 57: 8–21.
Putnam EA, Cho M, Zinn AB, Towbin JA, Byers PH, Milewicz DM : Delineation of the Marfan phenotype associated with mutations in exons 23-32 of the FBN1 gene. Am J Med Genet 1996; 62: 233–242.
Wang M, Price C, Han J et al: Recurrent mis-splicing of fibrillin exon 32 in two patients with neonatal Marfan syndrome. Hum Mol Genet 1995; 4: 607–613.
Faivre L, Collod-Beroud G, Loeys BL et al: Effect of mutation type and location on clinical outcome in 1,013 probands with Marfan syndrome or related phenotypes and FBN1 mutations: an international study. Am J Hum Genet 2007; 81: 454–466.
De Paepe A, Devereux RB, Dietz HC, Hennekam RC, Pyeritz RE : Revised diagnostic criteria for the Marfan syndrome. Am J Med Genet 1996; 62: 417–426.
Beighton P, De Paepe A, Steinmann B, Tsipouras P, Wenstrup RJ : Ehlers-Danlos syndromes: revised nosology, Villefranche, 1997. Ehlers-Danlos National Foundation (USA) and Ehlers-Danlos Support Group (UK). Am J Med Genet 1998; 77: 31–37.
Faivre L, Dollfus H, Lyonnet S et al: Clinical homogeneity and genetic heterogeneity in Weill-Marchesani syndrome. Am J Med Genet A 2003; 123: 204–207.
Fryns JP, Buttiens M : X-linked mental retardation with marfanoid habitus. Am J Med Genet 1987; 28: 267–274.
Greally MT, Carey JC, Milewicz DM et al: Shprintzen-Goldberg syndrome: a clinical analysis. Am J Med Genet 1998; 76: 202–212.
Loeys BL, Chen J, Neptune ER et al: A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat Genet 2005; 37: 275–281.
Furlong J, Kurczynski TW, Hennessy JR : New Marfanoid syndrome with craniosynostosis. Am J Med Genet 1987; 26: 599–604.
Collod-Beroud G, Lackmy-Port-Lys M, Jondeau G et al: Demonstration of the recurrence of Marfan-like skeletal and cardiovascular manifestations due to germline mosaicism for an FBN1 mutation. Am J Hum Genet 1999; 65: 917–921.
Shinawi M, Boileau C, Brik R, Mandel H, Bentur L : Splicing mutation in the fibrillin-1 gene associated with neonatal Marfan syndrome and severe pulmonary emphysema with tracheobronchomalacia. Pediatr Pulmonol 2005; 39: 374–378.
Collod-Beroud G, Le Bourdelles S, Ades L et al: Update of the UMD-FBN1 mutation database and creation of an FBN1 polymorphism database. Hum Mutat 2003; 22: 199–208.
Beroud C, Hamroun D, Collod-Beroud G, Boileau C, Soussi T, Claustres M : UMD (universal mutation database): 2005 update. Hum Mutat 2005; 26: 184–191.
Collod G, Beroud C, Soussi T, Junien C, Boileau C : Software and database for the analysis of mutations in the human FBN1 gene. Nucleic Acids Res 1996; 24: 137–140.
Liu WO, Oefner PJ, Qian C, Odom RS, Francke U : Denaturing HPLC-identified novel FBN1 mutations, polymorphisms, and sequence variants in Marfan syndrome and related connective tissue disorders. Genet Test 1997; 1: 237–242.
Wang M, Kishnani P, Decker-Phillips M, Kahler SG, Chen YT, Godfrey M : Double mutant fibrillin-1 (FBN1) allele in a patient with neonatal Marfan syndrome. J Med Genet 1996; 33: 760–763.
Downing AK, Knott V, Werner JM, Cardy CM, Campbell ID, Handford PA : Solution structure of a pair of calcium-binding epidermal growth factor-like domains: implications for the Marfan syndrome and other genetic disorders. Cell 1996; 85: 597–605.
Dietz HC, Pyeritz RE : Mutations in the human gene for fibrillin-1 (FBN1) in the Marfan syndrome and related disorders. Hum Mol Genet 1995; 4 (Spec No): 1799–1809.
Loeys B, Nuytinck L, Delvaux I, De Bie S, De Paepe A : Genotype and phenotype analysis of 171 patients referred for molecular study of the fibrillin-1 gene FBN1 because of suspected Marfan syndrome. Arch Intern Med 2001; 161: 2447–2454.
Comeglio P, Johnson P, Arno G et al: The importance of mutation detection in Marfan syndrome and Marfan-related disorders: report of 193 FBN1 mutations. Hum Mutat 2007; 28: 928.
Biggin A, Holman K, Brett M, Bennetts B, Ades L : Detection of thirty novel FBN1 mutations in patients with Marfan syndrome or a related fibrillinopathy. Hum Mutat 2004; 23: 99.
Rommel K, Karck M, Haverich A et al: Identification of 29 novel and nine recurrent fibrillin-1 (FBN1) mutations and genotype-phenotype correlations in 76 patients with Marfan syndrome. Hum Mutat 2005; 26: 529–539.
Loeys B, De Backer J, Van Acker P et al: Comprehensive molecular screening of the FBN1 gene favors locus homogeneity of classical Marfan syndrome. Hum Mutat 2004; 24: 140–146.
Hayward C, Porteous ME, Brock DJ : Mutation screening of all 65 exons of the fibrillin-1 gene in 60 patients with Marfan syndrome: report of 12 novel mutations. Hum Mutat 1997; 10: 280–289.
Matsukawa R, Iida K, Nakayama M et al: Eight novel mutations of the FBN1 gene found in Japanese patients with Marfan syndrome. Hum Mutat 2001; 17: 71–72.
Halliday DJ, Hutchinson S, Lonie L et al: Twelve novel FBN1 mutations in Marfan syndrome and Marfan related phenotypes test the feasibility of FBN1 mutation testing in clinical practice. J Med Genet 2002; 39: 589–593.
Katzke S, Booms P, Tiecke F et al: TGGE screening of the entire FBN1 coding sequence in 126 individuals with Marfan syndrome and related fibrillinopathies. Hum Mutat 2002; 20: 197–208.
Korkko J, Kaitila I, Lonnqvist L, Peltonen L, Ala-Kokko L : Sensitivity of conformation sensitive gel electrophoresis in detecting mutations in Marfan syndrome and related conditions. J Med Genet 2002; 39: 34–41.
Acknowledgements
We thank all physicians who referred patients for diagnosis and notably: H Plauchu (Lyon), Clarisse Baumann (Paris), Marlène Rio (Paris), Stanislas Lyonnet (Paris), and Didier Lacombe (Bordeaux). This study was supported in part by grants from GIS-maladies rares 2004; ANR-05-PCOD-014, Université Versailles Saint Quentin (Legs de Melle Bonnevie); Assistance Publique Hôpitaux de Paris (CIRC 2007); French Society of Cardiology and French Federation of Cardiology.33, 34, 35, 36, 37
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on European Journal of Human Genetics website (http://www.nature.com/ejhg)
Supplementary information
Rights and permissions
About this article
Cite this article
Stheneur, C., Collod-Béroud, G., Faivre, L. et al. Identification of the minimal combination of clinical features in probands for efficient mutation detection in the FBN1 gene. Eur J Hum Genet 17, 1121–1128 (2009). https://doi.org/10.1038/ejhg.2009.36
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejhg.2009.36
Keywords
This article is cited by
-
A population-based survey of FBN1 variants in Iceland reveals underdiagnosis of Marfan syndrome
European Journal of Human Genetics (2024)
-
Combining genetic constraint with predictions of alternative splicing to prioritize deleterious splicing in rare disease studies
BMC Bioinformatics (2022)
-
Genetic screening in heritable thoracic aortic disease—rationale, potentials and pitfalls
Indian Journal of Thoracic and Cardiovascular Surgery (2022)
-
A nonsense variant in FBN1 caused autosomal dominant Marfan syndrome in a Chinese family: a case report
BMC Medical Genetics (2020)
-
Skeletal evolution in Marfan syndrome: growth curves from a French national cohort
Pediatric Research (2018)