Abstract
Hyperglycemia impacts different vascular cell functions and promotes the development and progression of various vasculopathies including diabetic retinopathy. Although the increased rate of apoptosis in pericytes (PCs) has been linked to increased oxidative stress and activation of protein kinase C-δ (PKC-δ) and SHP-1 (Src homology region 2 domain-containing phosphatase-1) tyrosine phosphatase during diabetes, the detailed mechanisms require further elucidation. Here we show that the rate of apoptosis and expression of proapoptotic protein Bim were increased in the retinal PCs of diabetic Akita/+ mice and mouse retinal PCs cultured under high glucose conditions. Increased Bim expression in retinal PCs under high glucose conditions required the sustained activation of signal transducer and activator of transcription 1 (STAT1) through production of inflammatory cytokines. PCs cultured under high glucose conditions also exhibited increased oxidative stress and diminished migration. Inhibition of oxidative stress, PKC-δ or Rho-associated protein kinase I/II was sufficient to protect PCs against apoptosis under high glucose conditions. Furthermore, PCs deficient in Bim expression were protected from high glucose-mediated increased oxidative stress and apoptosis. However, only inhibition of PKC-δ lowered Bim levels. N-acetylcysteine did not affect STAT1 levels, suggesting that oxidative stress is downstream of Bim. PCs cultured under high glucose conditions disrupted capillary morphogenesis of retinal endothelial cells (ECs) in coculture experiments. In addition, conditioned medium prepared from PCs under high glucose conditions attenuated EC migration. Taken together, our results indicate that Bim has a pivotal role in the dysfunction of retinal PCs under high glucose conditions by increasing oxidative stress and death of PCs.
Similar content being viewed by others
Main
Dysfunction in retinal blood vessel and pathological neovascularization causes blindness in patients with diabetes.1, 2 Mechanisms of vascular dysfunction in diabetes have been the subject of numerous studies. A causal link between diabetic hyperglycemia and the development of retinal vascular complications is established, considering that tight glucose control in diabetic patients reduces the progression of the disease.3 Elucidating mechanisms for dysfunction of retinal vascular cell under high glucose conditions is necessary to develop new therapeutics for diabetic retinopathy.
Pericytes (PCs), smooth muscle-like cells that envelope capillaries provide vessel stability and control endothelial cell (EC) proliferation and survival. PCs have attracted much attention since the inception of studies to understand the pathogenesis of diabetic retinopathy. Alterations in the interactions between PCs and ECs have crucial roles in the development of diabetic retinopathy.4 Unfortunately, very little is known about the nature of these interactions, and how they are altered in diabetes.
PC loss is considered a hallmark of early diabetic retinopathy, which can result in focal EC proliferation associated with microaneurysm formation.5 Recent studies suggest that loss of PCs contributes not only to the vasodynamic changes in the early stages of diabetic retinopathy but also to neovascularization in proliferative diabetic retinopathy.6 In addition, several studies indicate that retinal PCs selectively degenerate to form PC ghosts, while ECs remain relatively constant, or pathologically proliferate when neovascularization forms.6, 7, 8 Although apoptosis of vascular cells is documented in the early stages of diabetic retinopathy, we believe that PCs sense and respond to hyperglycemia differently compared with ECs.
The BCL-2 protein family regulates apoptosis by maintaining mitochondrial homeostasis through a balanced activity of pro- and antiapoptotic family members.9 Among BCL-2 family of proteins, Bim is a proapoptotic protein with only one Bcl-2 homology (BH3) domain. Bim is required for the activation of cell death pathways mediated by other Bcl-2 proapoptotic family members, namely BAX and BAK.10 Bim expression is increased in the neuroretina of diabetic patients compared with non-diabetics.11 However, the role of Bim in the development and progression of diabetic retinopathy has not been addressed previously.
We recently described a novel method for isolation and culture of retinal PCs from wild-type and transgenic mice.12 Here we demonstrate that mouse retinal PCs not only exhibited higher rates of apoptosis, as shown in PCs from other species,13, 14 but also exhibited attenuation of migration under high glucose conditions. High glucose also had a significant impact on production of reactive oxygen species (ROS) and interaction of ECs and PCs during capillary morphogenesis. Furthermore, we uncovered that increased Bim expression required sustained activation of signal transducer and activator of transcription 1 (STAT1) through increased production of inflammatory cytokines. Furthermore, PCs prepared from Bim−/− mice were protected from high glucose ill effects. Collectively, our results suggest a pivotal role for Bim expression and/or activation in the dysfunction of PCs under high glucose conditions.
Results
Apoptosis of PCs was increased in the retina of diabetic mice and under high glucose conditions
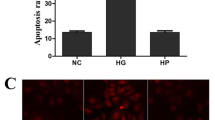
Apoptosis of PCs in mouse retina was determined in wild-type and Ins2Akita/+ mice with 7 months of diabetes. Akita/+ mice carry a mutation in their insulin gene and develop diabetes by 4 weeks of age. Diabetes is more prominent in male mice. These mice do exhibit many of the early non-proliferative change in their retina. In immunofluorescence staining for terminal deoxynucleotidyl transferase-mediated digoxigenin-deoxyuridine nick-end labeling (TUNEL) (apoptotic cells) and platelet-derived growth factor receptor-β (PDGFR-β) (PCs), an increase in TUNEL-positive PCs in diabetic retinas was observed compared with wild-type mice (Figure 1a). Quantification for TUNEL-positive PCs is shown in Figure 1b.
Diabetes induces apoptosis of PCs in retinal vasculature. (a) Retinal frozen sections were prepared from wild-type and Akita/+ mice. Double immunofluorescence staining was performed for PDGFR-β and TUNEL. Arrowheads indicate TUNEL- and PDGFR-β-positive cells in the superficial layer of the retina. Bar indicates 20 μm. (b) Quantification of TUNEL and PDGFR-β-positive cells is shown. Data are presented as mean±S.E.M. (n=7 for wild-type and n=8 for Akita/+ mice; *P<0.05 compared with wild-type mice). (c) The effects of high glucose on apoptosis of retinal PCs. Retinal PCs cultured under high glucose conditions exhibited a significantly higher rate of apoptosis. NG: normal glucose condition; HG: high glucose condition. Similar results were observed under fluctuation conditions (n≥3; *P≤0.05 (NG versus HG or fluctuation))
We next determined the impact of high glucose on retinal PC apoptosis in culture. The effect of high glucose on mouse retinal PCs has not been previously addressed. Retinal PCs were cultured in medium under normal, high glucose or osmolarity control for 5 days as detailed and previously used by us and others.15, 16, 17 Consistent with previous studies,13 mouse retinal PCs cultured under high glucose conditions exhibited a significantly higher rate of apoptosis compared with cells cultured under normal glucose or osmolarity control (Figure 1c). A similar rate of apoptosis to that observed under high glucose conditions was noted in PCs cultured under fluctuating glucose conditions (Figure 1c).
Bim level was increased in retinas of diabetic mice
We next examined whether expression of Bcl-2 family member Bim was altered in retinas of diabetic Ins2Akita/+ mice. Bim is a proapoptotic member of the Bcl-2 family with significant role in retinal vascular homeostasis.18 The expression level of Bim in mouse retina was determined in wild-type and Ins2Akita/+ mice. In immunofluorescence staining for Bim and PDGFR-β, an increase in Bim retinas was observed in diabetic compared with wild-type mice (Figure 2a). In superficial layer of retina, the expression level of Bim was increased in PCs. To further confirm increased Bim expression in diabetic mice retina, lysates from retinas were analyzed by western blotting. Bim level was significantly elevated in the retina of Ins2Akita/+ mice compared with wild-type mice (Figures 2b and c). In addition, the diabetic retina predominantly expressed the extra long form of Bim (BimEL), while non-diabetic retina expressed the shorter isoforms of Bim (BimL and BimS). Furthermore, BimEL is generally the prominent isoform when Bim level is upregulated.19
Bim expression was increased in the retina of diabetic mice. (a) Retinal frozen sections were prepared from wild-type and Akita/+ mice. Double immunofluorescence staining was performed for PDGFR-β and Bim. Arrowheads indicate Bim- and PDGFR-β-positive cells in the superficial layer of retina. Bar indicates 20 μm. (b) Expression of Bim in the retina of wild-type and Akita/+ mice was examined by western blot analysis. The antibody to β-catenin was used for loading control. (c) Quantification of band intensity in western blots (n≥4; *P≤0.05 (wild type versus Akita/+))
Oxidative stress is an upstream effector of high glucose-mediated PC dysfunction
Exposure of perivascular supporting cells to high glucose generally results in increased oxidative stress and apoptosis,13, 20, 21 perhaps through activation of protein kinase C-δ (PKC-δ) promoting diabetic retinopathy.13 To determine whether increased oxidative stress and/or activation of PKC-δ contributes similar to high glucose-induced apoptosis of mouse retinal PCs, we evaluated the rate of apoptosis in the presence of the N-acetylcysteine (NAC), an antioxidant with significant inhibitory effect on pathogenesis of diabetic retinopathy,22, 23 or Rottlerin. Rottlerin is a PKC-δ-specific inhibitor with an IC50 of 3–6 μM for PKC-δ.24, 25 Rottlerin inhibits apoptosis of smooth muscle cells in response to oxidative stress.26 Incubation with Rottlerin or NAC protected PCs from high glucose-mediated apoptosis (Figure 3a). Inhibition of SHP-1 (Src homology region 2 domain-containing phosphatase-1), as demonstrated previously,13 was also protective, although to a lesser extent (Figure 3a).
Signaling pathways involved in high glucose (HG)-mediated apoptosis and increased ROS production in PCs. (a) An increase in the rate of apoptosis was observed in PCs cultured under HG compared with normal glucose (NG) or osmolarity control (NG+L-Glu) conditions. Incubation of PCs under HG with NAC (antioxidant), Rottlerin (PKC-δ inhibitor) or Y-27632 (ROCK I/II inhibitor) reduced the rate of apoptosis. SSG (SHP-1 inhibitor) had a modest effect (n≥3; *P≤0.05 (NG versus HG); **P≤0.05 (HG versus NG+L-Glu or HG+inhibitors)). (b) Retinal PCs cultured under HG conditions exhibited a significantly elevated level of ROS compared with NG or NG+L-Glu conditions. Incubation of PCs under HG with NAC reduced the level of ROS. Inhibition of PKC-δ, SHP-1 and ROCK I/II had no significant effect on ROS levels under HG conditions (n≥30; *P≤0.05 (NG versus HG); **P≤0.05 (HG versus HG+NAC))
RhoA/Rho-associated protein kinase (ROCK) signaling pathway has an important role in regulation of angiogenesis, and its opposition by mitogen-activated protein kinase (MAPK)/extracellular signal-related kinase (ERK) signaling promotes EC survival and sprouting during angiogenesis.27, 28, 29 Very little is known about the contribution of RhoA/ROCK pathway to the function of PCs. We next determined the impact of ROCK I/II inhibitor, Y-27632, on PC apoptosis under high glucose conditions. Inhibition of ROCK I/II protected PCs from high glucose-induced apoptosis (Figure 3a). Thus, activation of RhoA/ROCK pathway makes a significant contribution to the proapoptotic phenotype of retinal PCs under high glucose conditions.
To examine the effect of high glucose condition on oxidative stress in PCs, the level of ROS was measured by dihydroethidium (DHE) staining. High glucose condition indeed elevated ROS levels in PCs. Osmolarity control conditions also increased ROS levels in PCs. This is consistent with a previous report showing that hyperosmotic stress elevates ROS levels.30 NAC inhibited ROS production under high glucose condition. In contrast, Rottlerin, sodium stibogluconate (SSG; an SHP-1-specific inhibitor31) and Y-27632 did not affect ROS production in PCs under high glucose conditions (Figure 3b). Thus, oxidative stress is an upstream effector of high glucose-mediating PC dysfunction.
High glucose enhanced Bim expression in retinal PCs
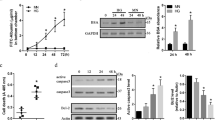
The BCL-2 protein family members have important roles in regulation of apoptosis and angiogenesis.18, 32 We also assessed p53 expression under various glucose conditions. The p53 expression was minimally affected in PCs cultured under various glucose conditions (Figure 4a). Although incubation of PCs with NAC under high glucose conditions had no significant effect on p53 expression, incubation of PCs with PKC-δ inhibitor resulted in a modest but significant increase in p53 expression under high glucose conditions.
Impact of high glucose (HG) on proapoptotic pathways. (a) Expression of p53 was confirmed by western blot analysis. The quantitative assessment of the data is also shown. No significant change in p53 expression was observed. NAC had no effect on the level of p53, while inhibition of PKC-δ caused a modest but significant increase in p53 level (n≥3; *P≤0.05 (normal glucose (NG) versus HG); **P≤0.05 (HG versus HG+Rottlerin)). (b) HG resulted in a significant increase in Bim expression compared with NG or osmolarity control (NG+L-Glu). Although incubation of PCs under HG with NAC had no significant effect on Bim expression, inhibition of PKC-δ (Rottlerin) resulted in significant decrease in Bim expression. (c) HG increased p-STAT1 level significantly compared with NG or NG+L-Glu. NAC and Rottlerin did not affect p-STAT1 level under HG condition. Graphs of band intensities from three independent experiments are also shown (n≥3; *P≤0.05 (NG versus HG); **P≤0.05 (HG versus HG+Rottlerin)). (d) Fludarabine, an inhibitor for STAT1, reduced the rate of apoptosis in PCs against apoptosis. HG condition increased the rate of apoptosis compared with NG and incubation of PCs with fludarabine under HG condition decreased the rate of apoptosis compared with HG conditions (n≥3; *P≤0.05 (NG versus HG and HG versus HG+Flu10))
We observed a significant increase in Bim expression of PCs cultured under high glucose conditions compared with normal glucose or osmolarity control (Figure 4b). Although incubation of PCs with NAC in high glucose did not have a significant effect on Bim expression, inhibition of PKC-δ reduced Bim expression to levels comparable to that seen in cells cultured in normal glucose. Expression of Bim in pancreatic β-cells is regulated by the activation of STAT1.33, 34 To elucidate the mechanism(s) for the upregulation of Bim expression, we examined the level of active and total STAT1 in PCs under various glucose conditions. The level of phosphorylated (p)-STAT1 in PCs cultured under high glucose conditions was significantly increased compared with normal glucose or osmolarity control (Figure 4c). Inhibition of ROS or PKC-δ under high glucose condition did not affect the level of p-STAT1.
We next examined if inhibition of STAT1 protects PCs from apoptosis induced by high glucose conditions. Fludarabine is a purine analog and potent inhibitor of STAT1 activity.35 Inhibition of STAT1 by fludarabine protected PCs against apoptosis under high glucose condition (Figure 4d). Thus, activation of STAT1, perhaps by production of inflammatory cytokines including tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) in PCs under high glucose conditions may occur. In fact, Supplementary Figures 1a and b show that incubation of PCs under high glucose conditions resulted in increased expression of TNF-α compared with normal glucose conditions.
We next examined which cytokines is required for PC apoptosis. Supplementary Figure 1C shows incubation of PCs with TNF-α-induced PC apoptosis, whereas monocyte chemotactic protein-1 (MCP-1) and IL-1β did not have any effect. The effect of inflammatory cytokines on the activation of STAT1 was then examined in PCs incubated with TNF-α and IL-1β under normal glucose conditions. TNF-α and IL-1β increased p-STAT1 level in retinal PCs (Supplementary Figure 1D). Thus, incubation of PCs under high glucose conditions results in the production of inflammatory cytokines, including TNF-α, and sustained activation of STAT1 driving Bim expression.
High glucose inhibited retinal PC migration
Migration of PCs is essential during vascular development and stabilization of newly formed vessels.36 Alteration in migration of PCs may also contribute to pathogenesis of DR.37 The impact of high glucose on migration of retinal PCs has not been previously addressed. We next assessed the migratory properties of PCs in transwell and scratch wound assays. We observed decreased PC migration under high glucose conditions compared with normal or control conditions in both scratch wound (Figure 5a) and transwell (Figure 5b) migration assays.
Attenuation of PC migration under high glucose (HG) conditions. (a) In scratch wound assays, a significant portion of wound remained uncovered in PCs cultured under HG compared with normal glucose (NG) or osmolarity control (NG+L-Glu) conditions. The quantitative assessment of the data is shown below (n≥3; *P≤0.05 (NG versus HG or HG versus NG+L-Glu)). (b) A significantly lower number of PCs cultured under HG conditions migrated through the transwell membrane compared with PCs cultured under NG or NG+L-Glu conditions (n≥3; *P≤0.05 (NG versus HG or HG versus NG+L-Glu)). (c) Incubation of PCs cultured under HG with NAC restored basal migration. Incubation of retinal PCs under HG conditions with Rottlerin (PKC-δ inhibitor) or Y-27632 (ROCK I/II inhibitor) restored basal migration to levels seen in PCs cultured under NG or NG+L-Glu conditions (n≥3; *P≤0.05 (NG versus HG), **P≤0.05 (HG versus NG+L-Glu or HG+NAC or HG+Rottlerin or HG+Y-27632). (d) Incubation of PCs with PDGF-BB enhanced their migration under NG conditions. However, PDGF-BB had a minimal effect on migration of PCs cultured under HG. Although PCK-α inhibitor (Gö6976) restored basal migration of PCs cultured in HG, it did not restore migratory response to PDGF-BB. The inhibition of SHP-1 (SSG) in PCs cultured in HG restored both basal and PDGF-stimulated migration (n≥3; *P≤0.05 (NG versus HG), **P≤0.05 (HG versus HG+Gö6976 or HG+SSG))
We next determined the downstream events that may contribute to the PC migratory defect under high glucose conditions. PCs incubated under high glucose conditions, in the presence of NAC, showed migration similar to that observed in PCs incubated under normal glucose or osmolarity control (Figure 5c). Similar results were observed in the presence of PKC-δ and ROCK I/II inhibitor (Figure 5c). Furthermore, retinal PCs cultured under normal glucose responded to promigratory activity of PDGF-BB, while basal and PDGF-BB-mediated migration of PCs under high glucose conditions was attenuated (Figure 5d). In contrast, PDGF stimulated the migration of PCs in high glucose in the presence of NAC (data not shown).
The activation of PKC-α also negatively impacts migration of SMC in response to PDGF.38 We also determined whether activation of PKC-α contributes to high glucose-mediated inhibition of retinal PC migration. Although inhibition of PKC-α restored basal migration of PCs under high glucose conditions, it minimally affected their PDGF-mediated migration (Figure 5d). Activation of SHP-1 tyrosine phosphatase is recently shown to impact negatively PDGF receptor signaling in retinal PCs under high glucose conditions.13 Inhibition of SHP-1 activity using the SHP-1-specific inhibitor SSG31, 39, 40 restored both basal and PDGF-mediated migration of PCs under high glucose conditions (Figure 5d).
Bim−/− PCs are protected from high glucose-mediated oxidative stress and apoptosis
Retinal PCs isolated from Bim−/− mice were used to investigate the function of PCs under normal or high glucose conditions. Apoptosis of Bim−/− PCs was not affected under high glucose conditions, whereas high glucose increased the apoptosis rate of wild-type PCs (Figure 6a). As indicated above, increased ROS production by high glucose is an upstream event in inducing apoptosis of PCs. High glucose condition did not impact ROS production in Bim−/− PCs, whereas ROS production in wild-type PCs was increased under high glucose conditions (Figure 6b).
Bim expression is essential for high glucose (HG)-mediated apoptosis and ROS production of PCs. (a) Bim−/− PCs were resistant to HG-induced apoptosis. Wild-type (WT) and Bim−/− PCs were incubated under normal glucose (NG) or HG conditions for 5 days. HG did not increase the rate of apoptosis in Bim−/− PCs (n≥3; *P≤0.05 (LG versus HG)). (b) Bim−/− PCs generated less ROS under HG conditions (n≥100; *P≤0.05 (NG versus HG)). (c) Migration of Bim−/− PCs was not affected under HG conditions, whereas the migration of WT PCs was attenuated under HG condition (n≥3; *P≤0.05 (NG versus HG))
Migratory property is crucial for the function of PCs in maintaining vascular structure and function. The migration of wild-type PCs was attenuated under high glucose condition compared with normal glucose conditions. However, the migration of Bim−/− PCs under high glucose conditions was not altered (Figure 6c). Thus, Bim expression may promote PC dysfunction under high glucose conditions.
PCs incubated under high glucose inhibited capillary morphogenesis and migration of ECs
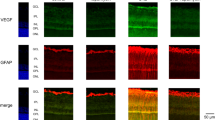
We recently showed that inflammatory mediators have a significant impact on capillary morphogenesis of retinal ECs.41 We next determined the effects of conditioned medium collected from PCs cultured under various glucose conditions on migration of retinal EC. Conditioned medium from PCs under high glucose conditions inhibited migration of ECs compared with PC conditioned medium under normal glucose or control (Figure 7a).
Incubation of PCs under high glucose (HG) conditions impacts their effect on EC function. (a) Conditioned medium was prepared from PCs incubated under normal glucose (NG), HG or control for 5 days. The effects of these conditioned medium on the migration of retinal EC was determined in a transwell assay. Please note a significant decrease in migration of retinal EC incubated with conditioned medium from PCs cultured in HG (n≥3; *P≤0.05 (NG versus HG and HG versus osmolarity control (NG+L-Glu)). (b) Retinal PCs were incubated under various glucose conditions for 5 days and used to assess their impact on retinal EC capillary morphogenesis in coculture (1 : 1) experiments. The mean number of branch points in 10 high power fields ( × 100) was determined. Please note a significant decrease in capillary morphogenesis of retinal ECs incubated with PCs under HG conditions (n≥3; *P≤0.05 (NG versus HG and HG versus NG+L-Glu)). Representative images for each condition are shown in (c)
ECs undergo capillary morphogenesis when grown on Matrigel, which mimics the late stage of angiogenesis.42 PCs were incubated under various glucose conditions and used in coculture experiments with retinal ECs. When ECs were mixed with PCs cultured under high glucose conditions, capillary morphogenesis of ECs was significantly inhibited compared with PCs cultured under normal glucose conditions (Figure 7b). Representative images of capillary morphogenesis are shown in Figure 7c.
Altered intracellular signaling pathways in retinal PCs cultured under high glucose conditions
Increased apoptosis of PCs under high glucose conditions is attributed to altered intracellular signaling pathways with important roles in cell survival, including Src/phosphoinositide 3 kinase, Akt, and MAPKs. We determined the activities of selected signaling molecules and pathways that impact PC function. Src phosphorylation was decreased in PCs under high glucose conditions compared with normal glucose or osmolarity control (Supplementary Figure 2A). Supplementary Figure 2B also shows decreased activation of Akt1 in PCs under high glucose and osmolarity control conditions compared with retinal PCs under normal glucose conditions. These results imply that osmotic challenge attenuates the activation of Akt1 in PCs as shown by others.17 In addition, activation of MAPK/ERKs, downstream effectors of Src kinase pathway, was also downregulated in retinal PCs under high glucose and osmolarity control compared with normal conditions (Supplementary Figure 2C). The levels of phosphorylated MAPK/p38 were not altered in PCs under high glucose conditions (Supplementary Figure 2D). In contrast, MAPK/c-Jun N-terminal kinase 1 (JNK1), whose activation is related to stress stimuli and increased apoptosis, was upregulated in PCs under high glucose conditions compared with normal glucose or control (Supplementary Figure 2E). Thus, high glucose conditions modulate various intracellular signaling pathways with significant impact on PC proliferation, migration and apoptosis.
Discussion
Here we used mouse retinal PCs to further delineate their response to high glucose and to elucidate the molecular mechanisms involved. Apoptosis of PCs was significantly increased under high glucose conditions and in the retina of diabetic Akita/+ mice. We showed that high glucose conditions increased Bim expression in PCs through the sustained activation of STAT1. The activation of STAT1 was mediated by the increased production of inflammatory cytokines including TNF-α under high glucose conditions. Bim is a proapoptotic protein and its level is significantly elevated in the neuroretina of diabetic patients.11 These results are consistent with increased Bim levels in the retina of diabetic Akita/+ mice and retinal PCs cultured under high glucose conditions reported here. Thus, increased Bim level in the retina, and more specifically in PCs, may be a crucial step in the development and progression of diabetic retinopathy.
To further demonstrate the important role of Bim in the apoptosis of PCs under high glucose conditions, we determined the apoptosis rate of retinal PCs prepared from Bim−/− mice under high glucose conditions. Bim deficiency protected PCs from apoptosis induced by high glucose conditions and reduced ROS levels. Thus, Bim expression contributes to ROS production under high glucose conditions and promotion of PC apoptosis. To elucidate how high glucose condition may increase Bim expression, we examined the level of p-STAT1 in PCs under various glucose conditions. STAT1 is a transcription factor and a known regulator of Bim expression, which is activated by proinflammatory cytokines including TNF-α, IL-1β, and interferon-γ.33, 34 We observed that high glucose conditions resulted in increased p-STAT1 level in PCs.
The increase in p-STAT1 level in PCs may result from increased level of proinflammatory cytokines produced by PCs under high glucose conditions. We observed that high glucose conditions significantly increased the mRNA level of TNF-α in PCs. High glucose condition is also shown to increase p-STAT1 level in renal tubular epithelial cell.43 Although NAC treatment under high glucose decreased p-STAT1 level in tubular epithelial cell, NAC treatment under high glucose did not affect the p-STAT1 level in PCs. This discrepancy may be attributed to the differences in duration of NAC treatment. Huang et al.43 treated renal tubular epithelial cells with NAC for 30 min under high glucose, but only that here PCs were exposed to NAC along with high glucose conditions for 5 days. Thus, chronic exposure to high glucose might contribute to sustained production of inflammatory mediators and activation of STAT1 in PCs and lack of response to NAC.
STAT1 has been considered as a drug target for vascular disease and fludarabine, a nucleoside analog, reduces STAT1 phosphorylation without affecting other STATs.44 We observed the protective effect of fludarabine against apoptosis induced by high glucose condition in PCs. Fludarabine also decreased Bim expression under high glucose conditions in PCs (not shown). Considering STAT1-mediated Bim expression and the role of Bim in the dysfunction of PCs under high glucose conditions, compounds affecting STAT1 expression and/or activity may alleviate the symptom of diabetic retinopathy.
Bim expression level is also regulated by various microRNAs (miRs). miR-24 is shown to attenuate apoptosis of cardiomyocytes by reducing Bim expression.45 In osteoblast, the miR cluster miR-17-92a inhibits apoptosis by reducing Bim expression level.46 However, the effect of high glucose or diabetic condition on regulation of miRNAs including miR-17-92a and miR-24 in retinal PCs remains unknown and is subject of current investigation in our laboratory.
The apoptosis of mouse retinal PCs cultured under high glucose conditions was also attenuated by the inhibition of downstream signaling events including PKC-δ, ROS production and ROCK I/II activity. The activation of these pathways under high glucose was previously shown to be responsible for PC dysfunction.13, 47, 48 Only NAC reduced ROS levels in PCs under high glucose, whereas the inhibition of PKC-δ, ROCK I/II or SHP-1 were ineffective. These results suggest that ROS production is an upstream event leading to the apoptosis of PCs under high glucose conditions. However, incubation of PCs with NAC under high glucose conditions did not affect Bim expression. Thus, Bim activity is upstream of ROS production.
Very few studies have systemically examined retinal PC migration under high glucose conditions. This property of PCs plays critical roles during angiogenesis and during the integrity of vascular function. Under high glucose conditions, the migration of wild-type PCs was significantly decreased. In contrast, high glucose had no significant effect on migration of Bim−/− PCs. Taken together, these results suggest that high glucose impairs PC migration and is impacted by Bim expression. Incubation of PCs with NAC, Rottlerin, Y-27632 and SSG under high glucose conditions restored PC migration, confirming the role of oxidative stress, PKC-δ, ROCK I/II and SHP-1 in PC migration.
PDGF-BB is secreted by EC promoting the recruitment of PCs to newly formed blood vessels and their stabilization.49 We showed that high glucose impaired PDGF-BB-mediated migration of PCs, which was reversed by inhibition of SHP-1. Thus, increased SHP-1 activity under high glucose conditions impairs not only PDGFR-β-mediated survival but also migration of PCs by reducing PDGFR-β activity.
Interactions between ECs and PCs are crucial for their survival and maintenance of vessel structure and function. To examine the effect of high glucose conditions on the interactions between retinal ECs and PCs, the effect of soluble factors released in the conditioned medium of PCs on migration of ECs was also investigated. Conditioned medium from PCs under high glucose conditions attenuated the migration of retinal ECs. We also examined the effect of PCs incubated under various glucose conditions on capillary morphogenesis of retinal ECs in coculture experiments. PCs are able to stabilize capillary tube formation by inhibiting tube regression in EC–PC cocultures.50 PCs cultured under high glucose conditions attenuated capillary morphogenesis of retinal ECs. This can be attributed to inhibition of EC migration by PCs cultured under high glucose, as demonstrated here. In addition, high glucose conditions may also disturb the expression of other regulating factors including metalloproteinases and tissue inhibitor of metalloproteinase.51 Thus, high glucose condition may affect secretion of soluble factors produced by PCs, including inflammatory cytokines that impact EC function as we recently demonstrated.41 The identity of these factors and their regulation under various glucose conditions require further investigation.
In summary, our results demonstrate that exposure of retinal PCs to high glucose results in increased Bim expression and oxidative stress, and reduced migration with a significant impact on their rate of apoptosis. We showed that these effects are associated with the activation of STAT1-mediated Bim expression axis in retinal PCs. A summary of how high glucose conditions affect signaling pathways involved in PC migration, proliferation, and apoptosis is shown in Figure 8. Collectively, we demonstrate that increased expression of Bim through the production of inflammatory cytokines and sustained activation of STAT1 is a significant pathway in the modulation of PC function under high glucose conditions. Thus, targeting Bim expression and/or activity may provide a more effective modality for treatment of diabetic retinopathy.
A summary of signaling pathways known to participate in response to high glucose. The exposure of PCs to high glucose results in increased Bim expression and oxidative stress, which lead to activation of SHP-1 and PKC-δ, and thus leading to enhanced apoptosis. Our results indicate that oxidative stress is upstream of SHP-1 and PKC-δ activation. In addition, we showed high glucose inhibited basal and PDGF-mediated migration of PCs and this can be reversed by NAC and inhibition PKC-δ or ROCKI/II
Materials and Methods
Animals
All experiments were carried out in accordance with and approved by the Institutional Animal Care and Use Committee of the University of Wisconsin School of Medicine and Public Health. Akita/+ heterozygous (Akita/+) male mice on C57BL/6J background were purchased from Jackson Laboratories (Bar Harbor, ME, USA). The diabetic (Akita/+) mice used in the experiments described here were male owing to effectiveness and severity of the diabetic phenotype in males. The wild-type male mice were used as control. Genomic tail DNA isolation and PCR were performed using the PCR Kit (REDExtract-N-Amp Tissue PCR Kit; Sigma, St. Louis, MO, USA), and the transgenic Ins2Akita/+ mice were identified by PCR screening. The amplified fragments were digested with Fnu4HI and resulting bands separated on 3.0% agarose gel, as recommended (Jackson Laboratories).
Immunohistochemical staining of the frozen sections. Frozen sections from mouse eyes were prepared as previously described by us.52 Seven-month-old mice were used for frozen sections. Apoptotic cell death was assessed by TUNEL staining. TUNEL staining was performed using Click-iT TUNEL Alexa Fluor Imaging Assay (Invitrogen, Carlsbad, CA, USA). Sections were then incubated with rabbit anti-PDGFRβ antibody (eBiosciences, San Diego, CA, USA) (1 : 500 dilution prepared in blocking solution) or rabbit anti-Bim antibody (Cell Signaling, Danvers, MA, USA) (1 : 500 dilution prepared in blocking solution) overnight at 4 °C in a humid environment. After three washes in phosphate-buffered saline (PBS) 5 min each, sections were incubated with appropriate CY3-conjugated secondary antibodies (Jackson ImmunoResearch, West Grove, PA, USA). Sections were counterstained with DAPI (Invitrogen) for staining of the nuclei for 15 min. Sections were washed three times with PBS, covered with PBS/glycerol (2 vol : 1 vol), and mounted with a coverslip. Sections were viewed by fluorescence microscopy, and images were captured in digital format using a Zeiss microscope (Carl Zeiss, Chester, VA, USA).
Cell culture
Mouse retinal PCs were isolated and cultured as previously described by us.12 The medium contained either 5.7 mM D-glucose (physiological/normal glucose; LG) or 40.7 mM D-glucose (hyperglycemia/high glucose; HG), or 5.7 mM D-glucose and 35 mM L-glucose (control; LG+L-Glu), as described previously.15 Cells were maintained in the growth medium for 5 days before each experiment, and the medium was changed every other day. The cells were also exposed to a cycle of high glucose for 2 days, normal glucose for 2 days, and then high glucose for 2 days before each experiment (fluctuation condition). Mouse retinal ECs were isolated and cultured as previously described by us.42
Apoptosis assays
Cells (0.1 ml of 1 × 105 cells per ml) were placed on fibronectin (2 μg/ml)-coated 4-well chamber slides. After attachment, cells were cultured in the medium under normal glucose or high glucose conditions or high glucose conditions containing various treatments such as the antioxidantNAC (1 mM; Sigma), PKC-δ inhibitor Rottlerin (1 μM; Sigma), ROCK1/2 inhibitor Y-27632 (10 μM; Cayman Chemical, Ann Arbor, MI, USA), SHP-1 inhibitor SSG (1 μM) (EMD, Gibbstown, NJ, USA) or fludarabine (10 μM; Sigma) for 5 days. Apoptotic cell death was assessed using Click-iT TUNEL Alexa Fluor Imaging Assay (Invitrogen), as recommended by the manufacturer. Positive cells were counted under fluorescence microscope and the percentage of apoptotic cells relative to the total number of cells was calculated. Concentrations for various pharmacological inhibitors were determined based on minimal effect on cell viability during the 5-day exposure to high glucose concentrations. The effect of cytokines on apoptosis was determined by measuring caspase activation using Caspase-Glo 3/7-assay kit (Promega, Madison, WI, USA), as recommended by the supplier. The assay provides caspase-3/7 DEVD-aminoluciferin substrate and the caspase-3/7 activity was detected by luminescent signal. For the assay, retinal PCs were plated at 8 × 103 cells per well of a 96-well plate and the next day incubated with 10 ng/ml TNF-α, IL-1β or MCP-1 for 24 h. Caspase activity was detected using a luminescent microplate reader (Victa2 1420 Multilabel Counter; Perkin-Elmer, Waltham, MA, USA). All samples were prepared in triplicate and repeated at least three times with similar results.
Determination of oxidative stress
Cells (0.1 ml of 1 × 105 cells per ml) were plated on fibronectin (2 μg/ml)-coated 4-well chamber slides. After attachment, cells were cultured in the medium under different glucose conditions for 5 days with/without various treatments as noted above. The level of cellular ROS was assessed using DHE staining (Invitrogen), as described previously.53 For quantitative assessments, the images were analyzed using Image J software (National Institutes of Health, Bethesda, MD, USA). Values were obtained from each cells captured in five high power fields ( × 400). More than 40 cells per each condition were analyzed.
Transwell migration assays
The impact of high glucose on migration of PCs and of conditioned medium collected from PCs under various glucose conditions on the migration of ECs was assessed using transwell assays as described in Scheef et al.12 The method for determining the effect of PDGF-BB on the migration of PCs was also described by us.12 To examine the migration of ECs, 1 × 105 cells in 100 μl of medium were added to the top of each transwell membrane. Transwell inserts were placed in 24-well dishes containing conditioned medium collected from PCs cultured under various glucose conditions for 5 days. Cells were allowed to migrate through the membrane for 3 h at 37 °C. The number of migrated cells was determined as described previously.15
Scratch wound assays
The migration of cells cultured under various glucose concentrations was also assessed by scratched wound assay as described.15 Confluent monolayers of retinal PCs cultured in different glucose conditions for 5 days were wounded using a 1-ml micropipette tip and incubated with growth medium with appropriate glucose concentration. Wound closure was monitored by phase microscopy, and the wounds were photographed at 0, 24, 48 and 96 h. Each sample was performed in triplicate on at least three independent occasions using two different isolations of PCs, with similar results.
Western blot analysis
Approximately 2 × 105 cells were plated in 60-mm plates and cultured in the growth medium containing different glucose concentrations for 5 days. For cytokine treatment, retinal PCs were incubated with 10 ng/ml TNF-α, IL-1β or 10 ng/ml TNF-α+10 ng/ml IL-1β for 24 h after serum starvation for 24 h. The cells were washed two times with cold PBS, lysed in 0.1 ml of lysis buffer (20 mM Tris (pH 7.4), 2 mM EDTA, 1 mM NaF, 1 mM Na3VO4, 1% Triton X-100, 1% NP-40, 0.1% sodium dodecyl sulfate (SDS) and protease inhibitor cocktail; Roche, Indianapolis, IN, USA) and briefly sonicated. The lysates were centrifuged and protein concentrations were determined using the BCA protein assay kit (Pierce, Rockford, IL, USA). For the retina lysate, the retinas were lysed in the same lysis buffer and prepared as described above for cells. The protein samples were mixed with appropriate volume of 6 × SDS sample buffer and analyzed using 4–20% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) (Invitrogen). Proteins were transferred to nitrocellulose membrane, blocked and incubated with appropriate primary antibodies at 4 °C overnight. After washing, the blots were incubated with appropriate HRP-conjugated secondary antibodies and developed using ECL system (GE Healthcare, Piscataway, NJ, USA). The antibody to β-actin (Sigma) or β-catenin (BD Biosciences, San Jose, CA, USA) was used to verify equal protein loading. The following primary antibodies were used: p-STAT1 (Tyr701), STAT1, p-Src (Tyr418), p-p38 (Thr180/Tyr182), p38, p-Akt1 (Ser 473), Akt1, p-ERK and ERK (Cell Signaling, Danvers, MA, USA), p-JNK1 and JNK1(R&D System, Minneapolis, MN, USA), Bim (Cell Signaling) and p53 (Santa Cruz Biotechnology, Santa Cruz, CA, USA).
Capillary morphogenesis
Capillary morphogenesis was performed as previously described by us.15 Equal numbers of retinal pericytes and ECs (2 × 105) in 2 ml were applied to the Matrigel-coated plates (0.5 ml Matrigel per 35 mm dish), incubated at 37 °C and photographed after 18 h in digital format using a Nikon microscope (Nikon Instruments, Melville, NY, USA). For quantitative assessment of the data, the mean number of branch points was determined by counting the number of branch points in five high-power fields ( × 100).
RNA purification and real-time qPCR analysis
The total RNA from PCs was extracted using mirVana PARIS Kit (Invitrogen). cDNA synthesis was performed from 1 μg of total RNA using Sprint RT Complete-Double PrePrimed Kit (Clontech, Mountain View, CA, USA). One microliter of each cDNA (dilution 1 : 10) was used as template in qPCR assays, performed in triplicate of three biological replicates on Mastercycler Realplex (Eppendorf, Hauppauge, NY, USA) using the SYBR-Green qPCR Premix (Clontech). Amplification parameters were as follows: 95 °C for 2 min; 40 cycles of amplification (95 °C for 15 s, 60 °C for 40 s); dissociation curve step (95 °C for 15 s, 60 °C for 15 s, 95 °C for 15 s). Primer sequences for TNF-α were: 5′-ACCGTCAGCCGATTTGCTAT-3′ (forward) and 5′-TTGACGGCAGAGAGGAGGTT-3′ (reverse). For IL-1β, 5′-GTTCCCATTAGACAACTGCACTACA-3′ (forward) and 5′-CCGACAGCACGAGGCTTTT-3′ (reverse). Standard curves were generated from known quantities for each of the target gene of linearized plasmid DNA. Ten times dilution series were used for each known target, which were amplified using SYBR-Green qPCR. The linear regression line for ng of DNA was determined from relative fluorescent units (RFU) at a threshold fluorescence value (Ct) to quantify gene targets from cell extracts by comparing the RFU at the Ct to the standard curve, normalized by the simultaneous amplification of RpL13a, a housekeeping gene. Primer sequences for RpL13a were: 5′-TCTCAAGGTTGTTCGGCTGAA-3′ (forward) and 5′-CCAGACGCCCCAGGTA-3′ (reverse).
Statistical analysis
Statistical differences between control and treated samples were evaluated with Student’s unpaired t-test (two-tailed) or one-way ANOVA with post hoc Dunnett’s test for multiple comparisons. Mean±S.E. are shown. P–values ≤0.05 were significant.
Abbreviations
- DHE:
-
dihydroethidium
- EC:
-
endothelial cell
- ERK:
-
extracellular signal-related kinase
- HG:
-
high glucose
- IL-1β:
-
interleukin-1β
- JNK:
-
c-Jun N-terminal kinase
- MAPK:
-
mitogen-activated protein kinase
- MCP-1:
-
monocyte chemotactic protein-1
- NAC:
-
N-acetylcysteine
- NG:
-
normal glucose
- PBS:
-
phosphate-buffered saline
- PC:
-
pericytes
- PDGFR-β:
-
platelet-derived growth factor receptor-β
- PKC:
-
protein kinase C
- ROCK:
-
Rho-associated protein kinase
- ROS:
-
reactive oxygen species
- SDS-PAGE:
-
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- SHP-1:
-
Src homology region 2 domain-containing phosphatase-1
- STAT:
-
signal transducer and activator of transcription
- TNF-α:
-
tumor necrosis factor-α
- TUNEL:
-
terminal deoxynucleotidyl transferase-mediated digoxigenin-deoxyuridine nick-end labeling
References
Gariano RF, Gardner TW . Retinal angiogenesis in development and disease. Nature 2005; 438: 960–966.
Frank RN . Diabetic retinopathy. N Engl J Med 2004; 350: 48–58.
The Diabetes Control and Complications Trial Research, G. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329: 977–986.
Gerhardt H, Betsholtz C . Endothelial–pericyte interactions in angiogenesis. Cell Tissue Res 2003; 314: 15–23.
Feng Y, vom Hagen F, Lin J, Hammes HP . Incipient diabetic retinopathy – insights from an experimental model. Ophthalmologica 2007; 221: 269–274.
Hammes HP, Feng Y, Pfister F, Brownlee M . Diabetic retinopathy: targeting vasoregression. Diabetes 2011; 60: 9–16.
Hayden MR, Yang Y, Habibi J, Bagree SV, Sowers JR . Pericytopathy: oxidative stress and impaired cellular longevity in the pancreas and skeletal muscle in metabolic syndrome and type 2 diabetes. Oxid Med Cell Longev 2010; 3: 290–303.
Aiello LP, Gardner TW, King GL, Blankenship G, Cavallerano JD, Ferris FL III et al. Diabetic retinopathy. Diabetes Care 1998; 21: 143–156.
Lomonosova E, Chinnadurai G . BH3-only proteins in apoptosis and beyond: an overview. Oncogene 2008; 27 (Suppl 1): S2–S19.
Ren D, Tu HC, Kim H, Wang GX, Bean GR, Takeuchi O et al. BID, BIM, and PUMA are essential for activation of the BAX- and BAK-dependent cell death program. Science 2010; 330: 1390–1393.
Valverde AM, Miranda S, Garcia-Ramirez M, Gonzalez-Rodriguez A, Hernandez C, Simo R . Proapoptotic and survival signaling in the neuroretina at early stages of diabetic retinopathy. Mol Vis 2013; 19: 47–53.
Scheef EA, Sorenson CM, Sheibani N . Attenuation of proliferation and migration of retinal pericytes in the absence of thrombospondin-1. Am J Physiol Cell Physiol 2009; 296: C724–C734.
Geraldes P, Hiraoka-Yamamoto J, Matsumoto M, Clermont A, Leitges M, Marette A et al. Activation of PKC-delta and SHP-1 by hyperglycemia causes vascular cell apoptosis and diabetic retinopathy. Nat Med 2009; 15: 1298–1306.
Han JH, Ha SW, Lee IK, Kim BW, Kim JG . High glucose-induced apoptosis in bovine retinal pericytes is associated with transforming growth factor beta and betaIG-H3: betaIG-H3 induces apoptosis in retinal pericytes by releasing Arg-Gly-Asp peptides. Clin Exp Ophthalmol 2010; 38: 620–628.
Huang Q, Sheibani N . High glucose promotes retinal endothelial cell migration through activation of Src, PI3K/Akt1/eNOS, and ERKs. Am J Physiol Cell Physiol 2008; 295: C1647–C1657.
Lin S, Sahai A, Chugh SS, Pan X, Wallner EI, Danesh FR et al. High glucose stimulates synthesis of fibronectin via a novel protein kinase C, Rap1b, and B-Raf signaling pathway. J Biol Chem 2002; 277: 41725–41735.
Varma S, Lal BK, Zheng R, Breslin JW, Saito S, Pappas PJ et al. Hyperglycemia alters PI3k and Akt signaling and leads to endothelial cell proliferative dysfunction. Am J Physiol 2005; 289: H1744–H1751.
Wang S, Park S, Fei P, Sorenson CM . Bim is responsible for the inherent sensitivity of the developing retinal vasculature to hyperoxia. Dev Biol 2011; 349: 296–309.
Gogada R, Yadav N, Liu J, Tang S, Zhang D, Schneider A et al. Bim, a proapoptotic protein, up-regulated via transcription factor E2F1-dependent mechanism, functions as a prosurvival molecule in cancer. J Biol Chem 2013; 288: 368–381.
King GL, Loeken MR . Hyperglycemia-induced oxidative stress in diabetic complications. Histochem Cell Biol 2004; 122: 333–338.
Kanwar M, Chan PS, Kern TS, Kowluru RA . Oxidative damage in the retinal mitochondria of diabetic mice: possible protection by superoxide dismutase. Invest Ophthalmol Vis Sci 2007; 48: 3805–3811.
Tsai GY, Cui JZ, Syed H, Xia Z, Ozerdem U, McNeill JH et al. Effect of N-acetylcysteine on the early expression of inflammatory markers in the retina and plasma of diabetic rats. Clin Exp Ophthalmol 2009; 37: 223–231.
El-Remessy AB, Behzadian MA, Abou-Mohamed G, Franklin T, Caldwell RW, Caldwell RB . Experimental diabetes causes breakdown of the blood-retina barrier by a mechanism involving tyrosine nitration and increases in expression of vascular endothelial growth factor and urokinase plasminogen activator receptor. Am J Pathol 2003; 162: 1995–2004.
Ryer EJ, Sakakibara K, Wang C, Sarkar D, Fisher PB, Faries PL et al. Protein kinase C delta induces apoptosis of vascular smooth muscle cells through induction of the tumor suppressor p53 by both p38-dependent and p38-independent mechanisms. J Biol Chem 2005; 280: 35310–35317.
Gschwendt M, Muller HJ, Kielbassa K, Zang R, Kittstein W, Rincke G et al. Rottlerin, a novel protein kinase inhibitor. Biochem Biophys Res Commun 1994; 199: 93–98.
Kato K, Yamanouchi D, Esbona K, Kamiya K, Zhang F, Kent KC et al. Caspase-mediated protein kinase C-δ cleavage is necessary for apoptosis of vascular smooth muscle cells. Am J Physiol 2009; 297: H2253–H2261.
Mavria G, Vercoulen Y, Yeo M, Paterson H, Karasarides M, Marais R et al. ERK-MAPK signaling opposes Rho-kinase to promote endothelial cell survival and sprouting during angiogenesis. Cancer Cell 2006; 9: 33–44.
Kroll J, Epting D, Kern K, Dietz CT, Feng Y, Hammes HP et al. Inhibition of Rho-dependent kinases ROCK I/II activates VEGF-driven retinal neovascularization and sprouting angiogenesis. Am J Physiol Heart Circ Physiol 2009; 296: H893–H899.
Bryan BA, Dennstedt E, Mitchell DC, Walshe TE, Noma K, Loureiro R et al. RhoA/ROCK signaling is essential for multiple aspects of VEGF-mediated angiogenesis. FASEB J 2010; 24: 3186–3195.
Hou T, Zhang X, Xu J, Jian C, Huang Z, Ye T et al. Synergistic triggering of superoxide flashes by mitochondrial Ca2+ uniport and basal reactive oxygen species elevation. J Biol Chem 2013; 288: 4602–4612.
Pathak MK, Yi T . Sodium stibogluconate is a potent inhibitor of protein tyrosine phosphatases and augments cytokine responses in hemopoietic cell lines. J Immunol 2001; 167: 3391–3397.
Wang S, Sorenson CM, Sheibani N . Attenuation of retinal vascular development and neovascularization during oxygen-induced ischemic retinopathy in Bcl-2−/− mice. Dev Biol 2005; 279: 205–219.
Moore F, Santin I, Nogueira TC, Gurzov EN, Marselli L, Marchetti P et al. The transcription factor C/EBP delta has anti-apoptotic and anti-inflammatory roles in pancreatic beta cells. PLoS One 2012; 7: e31062.
Barthson J, Germano CM, Moore F, Maida A, Drucker DJ, Marchetti P et al. Cytokines tumor necrosis factor-alpha and interferon-gamma induce pancreatic beta-cell apoptosis through STAT1-mediated Bim protein activation. J Biol Chem 2011; 286: 39632–39643.
Tajima K, Takaishi H, Takito J, Tohmonda T, Yoda M, Ota N et al. Inhibition of STAT1 accelerates bone fracture healing. J Orthop Res 2010; 28: 937–941.
Bjarnegård M, Enge M, Norlin J, Gustafsdottir S, Fredriksson S, Abramsson A et al. Endothelium-specific ablation of PDGFB leads to pericyte loss and glomerular, cardiac and placental abnormalities. Development 2004; 131: 1847–1857.
Armulik A, Abramsson A, Betsholtz C . Endothelial/pericyte interactions. Circ Res 2005; 97: 512–523.
Okazaki J, Mawatari K, Liu B, Kent KC . The effect of protein kinase C and its alpha subtype on human vascular smooth muscle cell proliferation, migration and fibronectin production. Surgery 2000; 128: 192–197.
Yi T, Pathak MK, Lindner DJ, Ketterer ME, Farver C, Borden EC . Anticancer activity of sodium stibogluconate in synergy with IFNs. J Immunol 2002; 169: 5978–5985.
Neviani P, Santhanam R, Trotta R, Notari M, Blaser BW, Liu S et al. The tumor suppressor PP2A is functionally inactivated in blast crisis CML through the inhibitory activity of the BCR/ABL-regulated SET protein. Cancer Cell 2005; 8: 355–368.
Palenski TL, Sorenson CM, Sheibani N . Inflammatory cytokine-specific alterations in retinal endothelial cell function. Microvas Res 2013; 89: 57–69.
Solowiej A, Biswas P, Graesser D, Madri JA . Lack of platelet endothelial cell adhesion molecule-1 attenuates foreign body inflammation because of decreased angiogenesis. Am J Pathol 2003; 162: 953–962.
Huang J-S, Chuang L-Y, Guh J-Y, Huang Y-J, Hsu M-S . Antioxidants attenuate high glucose-induced hypertrophic growth in renal tubular epithelial cells. Am J Physiol 2007; 293: F1072–F1082.
Torella D, Curcio A, Gasparri C, Galuppo V, De Serio D, Surace FC et al. Fludarabine prevents smooth muscle proliferation in vitro and neointimal hyperplasia in vivo through specific inhibition of STAT-1 activation. Am J Physiol Heart Circ Physiol 2007; 292: H2935–H2943.
Qian L, Van Laake LW, Huang Y, Liu S, Wendland MF, Srivastava D . miR-24 inhibits apoptosis and represses Bim in mouse cardiomyocytes. J Exp Med 2011; 208: 549–560.
Guo L, Xu J, Qi J, Zhang L, Wang J, Liang J et al. MicroRNA-17-92a upregulation by estrogen leads to Bim targeting and inhibition of osteoblast apoptosis. J Cell Sci 2013; 126: 978–988.
Xie X, Peng J, Chang X, Huang K, Huang J, Wang S et al. Activation of RhoA/ROCK regulates NF-κB signaling pathway in experimental diabetic nephropathy. Mol Cell Endocrinol 2013; 369: 86–97.
Mustapha NM, Tarr JM, Kohner EM, Chibber R . NADPH oxidase versus mitochondria-derived ROS in glucose-induced apoptosis of pericytes in early diabetic retinopathy. J Ophthalmol 2010; 2010: 746978.
Stratman AN, Schwindt AE, Malotte KM, Davis GE . Endothelial-derived PDGF-BB and HB-EGF coordinately regulate pericyte recruitment during vasculogenic tube assembly and stabilization. Blood 2010; 116: 4720–4730.
Saunders WB, Bohnsack BL, Faske JB, Anthis NJ, Bayless KJ, Hirschi KK et al. Coregulation of vascular tube stabilization by endothelial cell TIMP-2 and pericyte TIMP-3. J Cell Biol 2006; 175: 179–191.
Tarallo S, Beltramo E, Berrone E, Dentelli P, Porta M . Effects of high glucose and thiamine on the balance between matrix metalloproteinases and their tissue inhibitors in vascular cells. Acta Diabetol 2010; 47: 105–111.
Wu Z, Wang S, Sorenson CM, Sheibani N . Attenuation of retinal vascular development and neovascularization in transgenic mice over-expressing thrombospondin-1 in the lens. Dev Dyn 2006; 235: 1908–1920.
Tang Y, Scheef EA, Wang S, Sorenson CM, Marcus CB, Jefcoate CR et al. CYP1B1 expression promotes the proangiogenic phenotype of endothelium through decreased intracellular oxidative stress and thrombospondin-2 expression. Blood 2009; 113: 744–754.
Acknowledgements
This work was supported by Grants R01 EY016995, RC4 EY021357, P30EY016665, R21 EY023024 and P30 CA014520 UW Paul P Carbone Cancer Center Support Grant from the National Institutes of Health and an unrestricted departmental award from Research to Prevent Blindness. NS is a recipient of a Research Award from American Diabetes Association, 1-10-BS-160 and Retina Research Foundation. ESS is supported by a UW-Madison UW-Milwaukee Inter Campus grant and predoctoral fellowship from American Heart Association, 12PRE12030099.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by M Federici
Author contributions
ESS, QH and NS designed experiments and analyzed data. ESS, QH, ZG, TLP and IZ conducted experiments. CMS provided cells and animals. ESS, QH, CMS and NS contributed to writing the manuscript.
Supplementary Information accompanies this paper on Cell Death and Disease website
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Shin, E., Huang, Q., Gurel, Z. et al. STAT1-mediated Bim expression promotes the apoptosis of retinal pericytes under high glucose conditions. Cell Death Dis 5, e986 (2014). https://doi.org/10.1038/cddis.2013.517
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cddis.2013.517