Abstract
3-Acetyl-oleanolic acid (3Ac-OA) is a derivative of oleanolic acid (OA), which has shown therapeutic beneficial effects on diabetes and metabolic syndrome. In this study we investigated whether 3Ac-OA exerted beneficial effect on non-alcoholic fatty liver disease (NAFLD) in rats and its potential underlying mechanisms. Treatment with 3Ac-OA (1–100 μmol/L) dose-dependently decreased the intracellular levels of total cholesterol (TC) and triglyceride (TG) in FFA-treated primary rat hepatocytes and human HepG2 cell lines in vitro. Furthermore, oil red staining studies showed that 3Ac-OA caused dose-dependent decrease in the number of lipid droplets in FFA-treated primary rat hepatocytes. SD rats were fed a high fat diet (HFD) for 6 weeks and subsequently treated with 3Ac-OA (60, 30, 15 mg·kg-1·d-1) for 4 weeks. 3Ac-OA administration significantly decreased the body weight, liver weight and serum TC, TG, LDL-C levels in HFD rats. Furthermore, 3AcOA administration ameliorated lipid accumulation and cell apoptosis in the liver of HFD rats. Using adipokine array analyses, we found that the levels of 11 adipokines (HGF, ICAM, IGF-1, IGFBP-3, IGFBP-5, IGFBP-6, lipocalin-2, MCP-1, M-CSF, Pref-1 and RAGE) were increased by more than twofold in the serum of 3Ac-OA-treated rats, whereas ICAM, IGF-1 and lipocalin-2 had levels increased by more than 20-fold. Moreover, 3Ac-OA administration significantly increased the expression of glucose transporter type 2 (GLUT-2) and low-density lipoprotein receptor (LDLR), as well as the phosphorylation of AMP-activated protein kinase (AMPK), protein kinase B (AKT) and glycogen synthase kinase 3β (GSK-3β) in the liver tissues of HFD rats. In conclusion, this study demonstrates that 3Ac-OA exerts a protective effect against hyperlipidemia in NAFLD rats through AMPK-related pathways.
Similar content being viewed by others
Introduction
Non-alcoholic fatty liver disease (NAFLD) is considered to be the most common chronic liver disease and affects millions of people worldwide1. NAFLD has emerged as an integral part of metabolic syndrome and is associated with obesity, hyperlipidemia and diabetes2,3. NAFLD ranges from steatosis to the more aggressive non-alcoholic steatohepatitis (NASH)4. Several influencing factors, such as insulin resistance, lipid accumulation, inflammation and oxidative stress, have also been recognized as characteristic inducers of NAFLD5,6. Characterized by large fat droplet accumulation, NAFLD induces dysregulation of fatty acid metabolism, and the resulting imbalance between the infiux and efflux of fatty acid metabolism in the liver induces excessive lipid accumulation7. Significant overloading of FFA stimulates hepatic TG synthesis and directly causes hepatic lipotoxicity and infiammation8. Treatment strategies for NAFLD are mainly focused on dietary changes, enhancement of exercise levels and additional medication to ameliorate slight disease states. Bezafibrate, as a PPARα agonist, is a lipid-lowering agent that is used to treat hyperlipidemia2. However, when confronted with serious disease conditions, we lack effective treatment strategies. Therefore, a comprehensive and directed treatment for targeting hepatic lipid regulation remains to be developed.
Oleanolic Acid (OA) is a commonly used natural compound and exerts beneficial effects against diabetes and metabolic syndrome9,10. Our previous studies have showed that OA can inhibit GP (glycogen phosphorylase) and has beneficial effects against hypoglycemia in vivo 11,12,13. 3-Acetyl-Oleanolic Acid (3Ac-OA) is synthesized based on OA. We hypothesized that 3Ac-OA might have a therapeutic effect on NAFLD and ameliorate hyperlipidemia. The present study investigated the effects of 3Ac-OA on hyperlipidemia in NAFLD and clarified the mechanism involved both in vitro and in vivo.
Materials and methods
Chemicals and reagents
3Ac-OA (Figure 1) was obtained from the Center for Drug Discovery at the China Pharmaceutical University. All of the drugs used in the present study were dissolved in a 0.5% carboxymethylcellulose sodium (CMC-Na) solution. Plasma triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C) and hemoglobin Alc kits were purchased from Whitman Biotech (Nanjing, China). Antibodies (Abs) against LDLR and GLUT4 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA), and Abs against β-actin were purchased from Abcam (Cambridge, MA, USA). Abs against protein kinase B (PKB/Akt), phospho-Ser473-Akt (p-Akt), GSK3 and phospho-GSK3, and AMPK and phospho-AMPK were from Cell Signaling Technology (Beverly, MA, USA). HRP-conjugated secondary Abs, affinity-purified mouse anti-rabbit IgG and rabbit anti-mouse IgG were purchased from Sigma-Aldrich (St Louis, MO, USA). The adipokine array kit was purchased from R&D (St Paul, MN, USA). All other chemicals used were of reagent grade.
Culture and FFA treatment of HepG2 cells
Human hepatocellular carcinoma cells (HepG2) were obtained from American Type Culture Collection (ATCC, VA, USA). Cells were seeded and cultured in Dulbecco's Modified Eagle's Medium (DMEM) High Glucose with 10% FBS and a 1% Penicillin-Streptomycin solution at 37°C with 5% CO2. Cells were subsequently passaged with a 0.25% trypsin-EDTA solution. An FFA (0.5 mmol/L) mixture of oleate/palmitate at a ratio of 2:1 was used to establish a lipid accumulation model; the mixture was then added to DMEM medium containing a final concentration of 1% bovine serum albumin (BSA). At the same time, the cells were treated with 3Ac-OA (1, 10, 100 μmol/L); bezafibrate (100 μmol/L) was used as a positive control. The cellular neutral lipid droplet accumulation was measured by incubating the cells with Nile Red and measuring by FACS. The intracellular TG and TC levels of FFA-induced lipid storage within HepG2 cells was measured.
Isolation, culture and FFA treatment of primary rat hepatocytes
Primary rat hepatocytes isolated from male Sprague-Dawley (SD) rats (250–300 g) were cultured as previously described14. Primary hepatocytes were cultured in William E medium (Invitrogen, San Diego, CA, USA) containing 100 nmol/L insulin, 100 nmol/L dexamethasone, 100 IU/mL penicillin and 100 mg/mL streptomycin. The cells were then treated similarly to the HepG2 cells. Cell imaging by oil red O staining was used to measure the lipid contents. The intracellular TG and TC levels were measured.
Animals and treatments
Male Sprague Dawley rats (180–200 g) were purchased from the Shanghai Laboratory Animal Center (Shanghai, China). Animals were allowed free access to food and water throughout acclimatization and experimental periods. To examine the protective effects of 3Ac-OA on HFD-induced hyperlipidemia, rats were randomly assigned to the following six groups (n=8): (I) vehicle group (0.5% CMC-Na, normal diet); (II) HFD group (0.5% CMC-Na, high fat diet); (III) HFD plus atorvastatin (5 mg/kg); (IV) HFD plus 3Ac-OA 60 mg/kg; (V) HFD plus 3Ac-OA 30 mg/kg; and (VI) HFD plus 3Ac-OA 15 mg/kg. Rats in the vehicle group (I) were fed a standard diet; rats in the other five groups (II to VI) were fed a HFD for 6 weeks. The ingredients of the high fat diet included 2% cholesterol, 0.5% sodium cholate, 3% lard oil, 0.2% propylthiouracil and 94.3% basic fodder, which were mixed and irradiated with cobalt-60 (radiation dose 25.0 kGy) before use. The HFD was provided by the Jiangsu Xietong Biotech Company. Rats were treated daily with 3Ac-OA for 4 weeks. Then, rats were fasted for 16 h and sacrificed under anesthesia for collection of blood and liver samples. Rats were handled according to university and institutional legislation, regulated by the Committee for the Purpose of Control and Supervision of Experiments on Animals.
Histopathology analysis
Rat livers from different groups were collected and fixed in 4% paraformaldehyde in 0.1 mol/L PBS. Paraffin-embedded tissue regions (4 μm) were stained with hematoxylin and eosin (H&E) according to standard techniques. Samples were examined by a professional pathologist blinded to the treatment conditions to evaluate the levels of steatosis, inflammation and fibrosis.
Oil Red O staining
Oil red O staining and lipid droplet analysis were performed by cell imaging. Neutral lipid accumulation was determined by oil red O staining, which allows detection of TG and cholesterol esters. Frozen hepatic tissues were cut at 5–10 μm and mounted on slides, air-dried, and then fixed in ice-cold 10% formalin for 5–10 min. Slides were rinsed immediately in distilled water 3 times and then placed in absolute propylene glycol for 2–5 min to avoid carrying water into the Oil Red O step. Slides were stained in pre-warmed Oil Red O solution for 8–10 min in a 60 °C oven. Slides were then differentiated in an 85% propylene glycol solution for 2–5 min and rinsed twice in distilled water prior to staining in Mayer's hematoxylin for 30 s. After washing thoroughly in running tap water for 3 min, slides were placed in distilled water, mounted with glycerin jelly and observed by ImageJ software (National Institutes of Health Bethesda, MD, USA).
Nile Red staining
The lipid level in primary rat hepatocytes was determined using Nile Red. After cells were treated with FFA for 24 h, a monolayer of cells was washed twice with PBS, fixed with 3.7% formaldehyde for 10 min, and incubated for 3 min with a Nile Red and DAPI solution. Cells were then washed with PBS. The fluorescence level was determined using a fluorescence microscope. Image Pro plus software was used to quantify the fluorescence value.
Biochemical analysis
The serum lipid levels of TC, TG, LDL and HDL were investigated to evaluate the relative lipid content changes via commercially available kits provided by Whitman Biotech (Nanjing, China). The liver triglyceride and total cholesterol levels were analyzed according to the manufacturer's protocol. The liver index was calculated as follows and used to assess organ health and hepatic histological damage: Liver index=liver weight/body weight.
Electron microscopy
Ultra-thin sections of 60 nm tissues were fixed with 4% glutaraldehyde and treated as Wu described15. After rinsing in 0.1 mol/L phosphate buffer for 30 min, tissues were treated with 1% OsO4 for 1 h, dehydrated, and embedded with epoxy resin. Electron photomicrographs of the ultra-structures of tissue were taken under an electron microscope.
Rat adipokine array
Blood samples were clotted for 2 h at room temperature and then centrifuged for 20 min at 1000×g. Serum samples were assayed immediately or split and stored at ≤ -20°C, avoiding repeated freeze-thaw cycles. The relative expression levels of 30 rat adipokines were determined simultaneously by using an adipokine array following the protocol provided in the kit.
Western blotting
Lysis buffer [25 mmol/L Tris-HCl pH 7.5, 100 mmol/L NaCl, 2.5 mmol/L EDTA and EGTA, 20 mmol/L NaF, 1 mmol/L Na3VO4, 20 mmol/L Na β-glycerophosphate, 10 mmol/L Na pyrophosphate, 0.5% Triton X-100, 0.1% β-mercaptoethanol and a protease inhibitor cocktail (Roche)] was used to lyse tissues after various treatments. SDS-PAGE-resolved proteins were transferred to nitrocellulose membranes (GE Healthcare) and incubated with Abs to detect the proteins levels. After incubation with HRP-conjugated secondary Abs, signals were detected via ECL. All of the Western blot results are representative of at least two independent experiments.
Statistical analysis
Data are expressed as the mean±SD unless otherwise indicated. Two-tailed Student's t-tests and One-Way ANOVA were performed to determine statistically significant differences. A value of P<0.05 was considered significant at the 95% confidence level.
Results
3Ac-OA ameliorates FFA-induced lipid accumulation in primary rat hepatocytes and HepG2 cells
Primary rat hepatocytes and human HepG2 cells were cultured and induced with 0.5 mmol/L FFA with or without 3Ac-OA (1, 10, 100 μmol/L) for 24 h before measuring the total lipid levels. The results showed that 3Ac-OA decreased the intracellular TG level. Moreover, 3Ac-OA treatment exhibited a stronger effect than bezafibrate (100 μmol/L, positive control) in lowering TG and TC in a dose-dependent manner (Figure 2A, 2B: Human HepG2 cells; Figure 2C, 2D: Primary rat hepatocytes). Oil red O staining demonstrated that the FFA-induced group showed a marked increase in lipid levels compared with the control group (Figure 3A, 3B), whereas 3Ac-OA treatment greatly decreased the number of lipid droplets in a significant dose-dependent manner (Figure 3D, 3E, 3F). Furthermore, the high dose 3Ac-OA group exhibited the same effect as the bezafibrate group (positive control, Figure 3F). Nile red staining also showed a similar phenomenon. Compared with the control group, the FFA-treated group showed an increase in lipid accumulation, with 10 μmol/L 3Ac-OA treatment showing a greater effect in ameliorating lipid accumulation (Figure 4). All of the above results showed that the 3Ac-OA and bezafibrate treatment decreased the lipid accumulation induced by FFA.
Measurement of the intracellular TG and TC levels in FFA-induced HepG2 cells and primary rat hepatocytes (Model: 0.5 mmol/L FFA; bezafibrate: 100 μmol/L; 10-4: 3Ac-OA 100 μmol/L; 10-5: 3Ac-OA 10 μmol/L; 10-6: 3Ac-OA 1 μmol/L); (A) Measurement of the intracellular TG levels in FFA-induced lipid storage in HepG2 cells. (B) Measurement of the intracellular TC levels in FFA-induced lipid storage within HepG2 cells. (C) Measurement of the intracellular TG levels in FFA-induced lipid storage in primary rat hepatocytes. (D) Measurement of the intracellular TC levels in FFA-induced lipid storage in primary rat hepatocytes. ** P<0.01, compared with control group. # P<0.05, ## P<0.01, compared with model group.
Effects of 3Ac-OA on FFA-induced lipid storage in rat primary hepatocytes as detected by Oil red O staining (original magnification: ×100). (A) Control group. (B) Model group (0.5 mmol/L FFA). (C) 100 μmol/L bezafibrate+0.5 mmol/L FFA. (D) 1 μmol/L 3Ac-OA+0.5 mmol/L FFA. (E) 10 μmol/L 3Ac-OA+0.5 mmol/L FFA. (F) 100 μmol/L 3Ac-OA+0.5 mmol/L FFA.
Effects of 3Ac-OA on FFA-induced lipid storage in rat primary hepatocytes by Nile Red analysis. (A) Control group; (B) Model group (0.5 mmol/L FFA); (C) 100 μmol/L bezafibrate+0.5 mmol/L FFA; (D) 1 μmol/L 3Ac-OA+0.5 mmol/L FFA; (E) 10 μmol/L 3Ac-OA +0.5 mmol/L FFA; (F) 100 μmol/L 3Ac-OA+0.5 mmol/L FFA.
3Ac-OA decreases food consumption, body weight, liver weight and serum biochemical parameters in HFD-treated rats
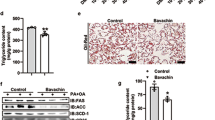
To determine whether 3Ac-OA had anti-steatotic effects in vivo, rats were fed a vehicle (0.5% CMC-Na) and HFD containing 2% cholesterol once daily by intragastric administration for 6 weeks, followed by treatment with 3Ac-OA for 4 weeks. Compared with the control group, the HFD group showed a significantly lower average weight and food intake, but marked lipid accumulation and histological indications. In the process of raising the rats, we observed that rats fed a HFD had no appetite because of the food was full of oil. However, the TC, TG and LDL levels were significantly increased in the HFD group. Treatment of rats with 3Ac-OA at 30 or 60 mg/kg per day for 4 weeks significantly decreased the body weight. There was no significant difference in the daily food intake between the 3Ac-OA-treated and control groups. The 3Ac-OA treatment markedly decreased the levels of serum TC, TG and LDL in a dose-dependent manner. Although the 3Ac-OA treatment did not show a significant effect on the HDL level in a dose-dependent manner, it still produced a superior result than atorvastatin. Furthermore, the liver triglyceride and cholesterol levels were reduced after 3Ac-OA treatment. By contrast, treatment with atorvastatin in rats fed a HFD for 4 weeks resulted in no change in these parameters (Table 1). In our previous studies, 3Ac-OA (60 mg/kg) was given to normal rats for 4 weeks, and no obvious toxicity was observed. The behavior, food/water intake, body weight, and plasma TC and TG levels of rats had no significant differences compared with control rats, which showed that 3Ac-OA at 60 mg/kg has no effect on these parameters in normal rats.
3Ac-OA ameliorates HFD-induced lipid accumulation and cell apoptosis in rats
Hematoxylin and eosin (H&E) staining showed that the HFD group had significant inflammation, infiltration of lymphocytes and macrophages, steatosis and lipid droplet accumulation compared with the control group (Figure 5A, 5B). 3Ac-OA treatment significantly ameliorated the above symptoms (Figure 5D, 5E, 5F). Furthermore, the 3Ac-OA treatment with the highest dose showed minimal infiammatory cell infiltrates and only a small amount of hepatocyte degeneration in rats, superior to that of the atorvastatin treatment group (Figure 5C). Oil red O staining also showed marked lipid accumulation in the HFD-fed group compared with the control group (Figure 6A, 6B). Treatment with 3Ac-OA resulted in few cytoplasmic alterations in hepatocytes, and the lipid droplet levels also decreased (Figure 6D, 6E, 6F). We further used electron microscopy to analyze the cell apoptosis and lipid accumulation induced by HFD. Compared to the control group (Figure 7A), cells displayed disruption of nuclear integrity and lysosomal structures, chromatin margination and condensation in the HFD model group (Figure 7B). There were significant fat vacuoles in the model group. Atorvastatin showed beneficial effects in maintaining cell integrity (Figure 7C). 3Ac-OA significantly inhibited the HFD-induced lipid accumulation and reduced cell apoptosis (Figure 7D, 7E, 7F).
Ac-OA alters the secretion of multiple adipokines in HFD-induced rats
Adipokine array analyses showed that the secretion of multiple adipokines was altered by treatment with 3Ac-OA. The levels of eleven adipokines were increased by more than two-fold in the serum of 3Ac-OA-treated rats, including HGF, ICAM, IGF-1, IGFBP-3, IGFBP-5, IGFBP-6, Lipocalin-2, MCP-1, M-CSF, Pref-1 and RAGE (Figure 8, Table 2). Several adipokines, such as ICAM, IGF-1, and Lipocalin-2, had levels increased by more than twenty-fold. Most of the adipokines were associated with insulin-like growth factors.
3Ac-OA activates AMPK-related pathways
We also examined the effects of 3Ac-OA on energy metabolism signaling pathways. The HFD model group showed a decrease in expression of LDLR, GLUT-2 (Figure 9A), phospho-AKT, phospho-GSK-3β and phospho-AMPK. The 3Ac-OA treatment group rescued phosphorylation of AMPK, GSK-3β and AKT and also rescued expression of LDLR and GLUT2 compared with the HFD-induced group (Figure 9B).
(A–B) Representative western blots of total protein extracts from the control group, HFD group, Atorvastatin group, 3Ac-OAH group (60 mg/kg), 3Ac-OAM group (30 mg/kg), and 3Ac-OAL group (15 mg/kg); n=6 in each experimental group. (C–D) The fold-change was calculated based on the densitometric analysis of band intensities. ** P<0.01 vs model group.
Discussion
Epidemiological studies have indicated that liver disease remains a major health problem throughout the world. Non-alcoholic fatty liver disease and its subtype non-alcoholic steatohepatitis affect approximately 35% of the population worldwide16. Half of all deaths in patients with non-alcoholic fatty liver disease are due to cardiovascular disease and malignancy, and yet overall disease awareness remains low. Despite new breakthroughs in hepatology, modern medicine lacks a reliable hepato-protective drug with few side effects17. Additionally, liver injury is one of the major side effects of many potential candidates, resulting in numerous failures of new drugs18,19.
It is well-known that an imbalance between TG excretion, synthesis and hepatic uptake of FFA will result in fatty liver disease. In hepatocytes, excess TG accumulation results in FFA esterification and uptake20, whereas FFA and lipid metabolites potently cause lipoapoptosis and lipotoxicity. High concentrations of circulating FFA disrupt lipid metabolism in NAFLD patients, which may aggravate hepatic fat accumulation. Regulating hepatic FFA metabolism is a conceivable strategy to treat early-stage NAFLD by stimulating FFA oxidation or decreasing FFA synthesis21. FFA is the key player in the pathogenesis of NAFLD by influencing metabolic regulators22. In this study, FFA was used to induce lipid accumulation in primary rat hepatocytes and HepG2 cells, and 3Ac-OA reduced the levels of TC and TG at the cellular level. Furthermore, we established a NAFLD model by using HFD-fed rats. 3Ac-OA improved the metabolic and histologic effects in HFD-treated rats. Oil Red O staining and electron microscopy also showed similar results, and 3Ac-OA significantly inhibited cell apoptosis by improving nuclear disruption, the structural integrity of lysosomes, chromatin margination and condensation, and lipid accumulation in HFD-induced rats.
The present study demonstrated the protective effects of 3Ac-OA on FFA-induced lipotoxicity in vitro in HepG2/primary rat hepatocytes and in vivo in HFD-induced hyperlipidemia NAFLD models for the first time. Animals fed a HFD had a higher caloric intake and developed NAFLD to similar pathological degrees compared to rats in the control group. However, caloric intake in rats among the HFD fed groups did not show significant differences with other groups. Serum biochemical index analysis showed a significant increases in the TG, TC, HDL-C and LDL-C levels of 49%, 195%, 46% and 184%, respectively. 3Ac-OA showed an extremely significant efficacy in reducing the TG and LDL-C levels, superior to the atorvastatin-treated group. Furthermore, similar pathological changes in NAFLD were induced in HFD-fed rats. The histopathological results showed that hepatocytes in the HFD-induced group underwent macrovascular cytoplasmic alterations. After treatment with 3Ac-OA, these changes were reversed in a dose-dependent manner, supporting that triterpenes have a positive hypolipidemic effect.
HFD-treated animals showed dyslipidemia and hepatic lipid accumulation; on the other hand, continuous HFD treatment induced oxidative infiltration and adipokine factors in the liver, which may develop to NASH. Similarly, the present investigation showed that rats with HFD-induced NAFLD exhibited higher levels of oxidative stress factors, lipid peroxidation products, and pro-infiammatory cytokines. In this study, we used a rat adipokine array kit to measure the relative expression levels of adipokines in serum. The results showed that 3Ac-OA increased secretion of HGF, ICAM-1, IGF-1, IGFBP-5, IGFBP-3, IGFBP-6, Lipocalin-2, MCP-1, M-CSF, Pref-1 and RAGE compared with the model group. Several adipokines, such as IGF-1, IGFBP-5, IGFBP-3, and IGFBP-6, were derived from insulin growth factors. IGF-I, which is mostly carried by IGF-binding protein 3 (IGFBP-3) in blood, was associated with cancer, diabetes and chronic kidney disease in a previous multiethnic study23. In this study, there were several records suggesting that IGFBP-3 may be partly associated with lipid metabolism. Further details of the effects of the mechanism of 3Ac-OA on IGF pathways are under study .
AMPK plays an important role in maintaining energy and redox homeostasis under various conditions24. A well-known role of AMPK is its participation in the regulation of many metabolic processes. In muscle cells, AMPK is important in the processes of glucose uptake and fatty acid oxidation; in the liver, AMPK plays a key role in fatty acid synthesis and gluconeogenesis25,26,27. Due to its roles in energy regulation, the AMPK pathway is regarded as a potential therapeutic target for obesity and diabetes. The HFD model group showed a marked decrease in expression of AKT, GSK-3β and AMPK. The signaling pathways of hepatic insulin and obesity are impaired by HFD exposure. In C2C12 cells, AMPK directly stimulates IRS-associated PI3K and the downstream signaling factors Akt and GSK-3β to ensure normal metabolism28. The 3Ac-OA treatment group had increased phosphorylation of AMPK and AKT compared to the untreated HFD group. 3Ac-OA might exert its modulatory effects on NAFLD through multiple signaling pathways involving AMPK. Further studies are needed to address the detailed molecular mechanisms in vitro.
This project was partially aimed at drug discovery based on pharmacological interference with lipid metabolism. We previously focused on research and development of pentacyclic triterpenes as natural and low-toxic anti-diabetic agents, which are used to provide preventive and therapeutic effects against non-alcoholic fatty liver complications. The present study provides the first evidence for the protective effects of 3Ac-OA in FFA/HFD-induced lipotoxicity both in vivo in NAFLD models and in vitro in HepG2/primary rat hepatocytes. 3Ac-OA may hold great promise as a potential natural therapeutic agent for the treatment of NAFLD.
Author contribution
Jun LIU designed the research, contributed to the execution of the research, analyzed the data and wrote the manuscript. Qiong OU-YANG, Chun-xiao XUAN and Xue WANG performed the research; Han-qiong LUO, Jin-E LIU and Lan-lan WANG provided the materials and protocols. Ting-ting LI and Yu-peng CHEN performed the experiments and analyzed the data.
Abbreviation
NAFLD: non-alcoholic fatty liver disease; 3Ac-OA: 3-Acetyl-Oleanolic Acid; OA: oleanolic acid; HFD: high fat diet; TC: total cholesterol; TG: triglyceride; HDL-C: high-density lipoprotein-cholesterol; LDL-C: low-density lipoprotein-cholesterol; FFA: free fatty acids; GP: glycogen phosphorylase; HGF: hepatocyte growth factor; ICAM-1: intercellular cell adhesion molecule-1; IGF-I: insulin-like growth factor I; IGFBP-3: IGF-binding protein 3; IGFBP-5: IGF-binding protein 5; IGFBP-6: IGF-binding protein 6; MCP-1: monocyte chemotactic protein 1; M-CSF: macrophage colony-stimulating factor; Pred-1: pre-adipocyte factor 1; GLUT-2: glucose transporter type 2; LDLR: low-density lipoprotein receptor; AMPK: AMP-activated protein kinase; AKT: protein kinase B; GSK-3β: glycogen synthase kinase 3β.
References
Takahashi Y, Sugimoto K, Inui H, Fukusato T. Current pharmacological therapies for nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol 2015; 21: 3777–85.
Tilg H, Hotamisligil GS. Nonalcoholic fatty liver disease: Cytokine-adipokine interplay and regulation of insulin resistance. Gastroenterology 2006; 131: 934–45.
Barritt ASt, Dellon ES, Kozlowski T, Gerber DA, Hayashi PH. The influence of nonalcoholic fatty liver disease and its associated comorbidities on liver transplant outcomes. J Clin Gastroenterol 2011; 45: 372–8.
Piccinin E, Moschetta A. Hepatic-specific PPARalpha-FGF21 action in NAFLD. Gut 2016; 65: 1075–6.
Miyake T, Kumagi T, Hirooka M, Furukawa S, Yoshida O, Koizumi M, et al. Low alcohol consumption increases the risk of impaired glucose tolerance in patients with non-alcoholic fatty liver disease. J Gastroenterol 2016; 51: 1090–100.
Arrese M, Cabrera D, Kalergis AM, Feldstein AE. Innate immunity and inflammation in NAFLD/NASH. Dig Dis Sci 2016; 61: 1294–303.
Chen S, Wen X, Zhang W, Wang C, Liu J, Liu C. Hypolipidemic effect of oleanolic acid is mediated by the miR-98-5p/PGC-1beta axis in high-fat diet-induced hyperlipidemic mice. FASEB J 2016; 31: 1085–96.
Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest 2005; 115: 1343–51.
Camer D, Yu Y, Szabo A, Huang XF. The molecular mechanisms underpinning the therapeutic properties of oleanolic acid, its isomer and derivatives for type 2 diabetes and associated complications. Mol Nutr Food Res 2014; 58: 1750–9.
Jiang Q, Wang D, Han Y, Han Z, Zhong W, Wang C. Modulation of oxidized-LDL receptor-1 (LOX1) contributes to the antiatherosclerosis effect of oleanolic acid. Int J Biochem Cell Biol 2015; 69: 142–52.
Zhang L, Dong J, Zhang Y, Liu J, Sun H. Design, synthesis, and biological evaluation of potent photoaffinity probes of oleanolic acid. Med Chem 2013; 9: 294–302.
Wang X, Chen Y, Abdelkader D, Hassan W, Sun H, Liu J. Combination therapy with oleanolic acid and metformin as a synergistic treatment for diabetes. J Diabetes Res 2015; 2015: 973287.
Cheng K, Liu J, Liu X, Li H, Sun H, Xie J. Synthesis of glucoconjugates of oleanolic acid as inhibitors of glycogen phosphorylase. Carbohydr Res 2009; 344: 841–50.
Hong EJ, Levasseur MP, Dufour CR, Perry MC, Giguere V. Loss of estrogen-related receptor alpha promotes hepatocarcinogenesis development via metabolic and inflammatory disturbances. Proc Natl Acad Sci U S A 2013; 110: 17975–80.
Wu X, Zhang L, Gurley E, Studer E, Shang J, Wang T, et al. Prevention of free fatty acid–induced hepatic lipotoxicity by 18β-glycyrrhetinic acid through lysosomal and mitochondrial pathways. Hepatology 2008; 47: 1905–15.
Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA 2015, 313: 2263–73
Ferslew BC, Johnston CK, Tsakalozou E, Bridges AS, Paine MF, Jia W, et al. Altered morphine glucuronide and bile acid disposition in patients with nonalcoholic steatohepatitis. Clin Pharmacol Ther 2015; 97: 419–27.
Lukacs-Kornek V, Schuppan D. Dendritic cells in liver injury and fibrosis: shortcomings and promises. J Hepatol 2013; 59: 1124–6.
Tarantino G, Citro V, Finelli C. Recreational drugs: a new health hazard for patients with concomitant chronic liver diseases. J Gastrointestin Liver Dis 2014; 23: 79–84.
Ibrahim SH, Kohli R, Gores GJ. Mechanisms of lipotoxicity in NAFLD and clinical implications. J Pediatr Gastroenterol Nutr 2011; 53: 131–40.
Wang JW, Wan XY, Zhu HT, Lu C, Yu WL, Yu CH, et al. Lipotoxic effect of p21 on free fatty acid-induced steatosis in L02 cells. PLoS One 2014; 9: e96124.
Chu JH, Wang H, Ye Y, Chan PK, Pan SY, Fong WF, et al. Inhibitory effect of schisandrin B on free fatty acid-induced steatosis in L-02 cells. World J Gastroenterol 2011; 17: 2379–88.
Romaniuk D, Kimsa MW, Strzalka-Mrozik B, Kimsa MC, Kabiesz A, Romaniuk W, et al. Gene expression of IGF1, IGF1R, and IGFBP3 in epiretinal membranes of patients with proliferative diabetic retinopathy: preliminary study. Mediators Inflamm 2013; 2013: 986217.
Jeon SM, Hay N. The double-edged sword of AMPK signaling in cancer and its therapeutic implications. Arch Pharm Res 2015; 38: 346–57.
King TS, Russe OQ, Moser CV, Ferreiros N, Kynast KL, Knothe C, et al. AMP-activated protein kinase is activated by non-steroidal anti-inflammatory drugs. Eur J Pharmacol 2015; 762: 299–305.
Basta HA, Palmenberg AC. AMP-activated protein kinase phosphorylates EMCV, TMEV and SafV leader proteins at different sites. Virology 2014; 462-463: 236–40.
Landgraf RR, Goswami D, Rajamohan F, Harris MS, Calabrese MF, Hoth LR, et al. Activation of AMP-activated protein kinase revealed by hydrogen/deuterium exchange mass spectrometry. Structure 2013; 21: 1942–53.
Brabant G, Muller G, Horn R, Anderwald C, Roden M, Nave H. Hepatic leptin signaling in obesity. FASEB J 2005; 19: 1048–50.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No 81673443). We also thank Dr Hong-bin SUN for providing compounds.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ou-Yang, Q., Xuan, Cx., Wang, X. et al. 3-Acetyl-oleanolic acid ameliorates non-alcoholic fatty liver disease in high fat diet-treated rats by activating AMPK-related pathways. Acta Pharmacol Sin 39, 1284–1293 (2018). https://doi.org/10.1038/aps.2017.142
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2017.142
Keywords
This article is cited by
-
Therapeutic potential of oleanolic acid in liver diseases
Naunyn-Schmiedeberg's Archives of Pharmacology (2024)
-
Metformin induces pyroptosis in leptin receptor-defective hepatocytes via overactivation of the AMPK axis
Cell Death & Disease (2023)
-
Linalool Mitigated High-Fat Diet–Induced Non-alcoholic Fatty Liver Disease by Regulating the Intestinal-Hepatic Axis via TGF-β/NF-kB/TLR4/ZO-1 Pathway
Revista Brasileira de Farmacognosia (2023)