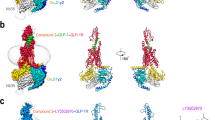
Abstract
Clinical studies indicate that partial agonists of the G-protein-coupled, free fatty acid receptor 1 GPR40 enhance glucose-dependent insulin secretion and represent a potential mechanism for the treatment of type 2 diabetes mellitus. Full allosteric agonists (AgoPAMs) of GPR40 bind to a site distinct from partial agonists and can provide additional efficacy. We report the 3.2-Å crystal structure of human GPR40 (hGPR40) in complex with both the partial agonist MK-8666 and an AgoPAM, which exposes a novel lipid-facing AgoPAM-binding pocket outside the transmembrane helical bundle. Comparison with an additional 2.2-Å structure of the hGPR40–MK-8666 binary complex reveals an induced-fit conformational coupling between the partial agonist and AgoPAM binding sites, involving rearrangements of the transmembrane helices 4 and 5 (TM4 and TM5) and transition of the intracellular loop 2 (ICL2) into a short helix. These conformational changes likely prime GPR40 to a more active-like state and explain the binding cooperativity between these ligands.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Choi, Y.J., Shin, D. & Lee, J.Y. G-protein coupled receptor 40 agonists as novel therapeutics for type 2 diabetes. Arch. Pharm. Res. 37, 435–439 (2014).
Kotarsky, K., Nilsson, N.E., Flodgren, E., Owman, C. & Olde, B. A human cell surface receptor activated by free fatty acids and thiazolidinedione drugs. Biochem. Biophys. Res. Commun. 301, 406–410 (2003).
Flodgren, E. et al. GPR40 is expressed in glucagon producing cells and affects glucagon secretion. Biochem. Biophys. Res. Commun. 354, 240–245 (2007).
Shapiro, H., Shachar, S., Sekler, I., Hershfinkel, M. & Walker, M.D. Role of GPR40 in fatty acid action on the beta cell line INS-1E. Biochem. Biophys. Res. Commun. 335, 97–104 (2005).
Lin, D.C. et al. Identification and pharmacological characterization of multiple allosteric binding sites on the free fatty acid 1 receptor. Mol. Pharmacol. 82, 843–859 (2012).
Srivastava, A. et al. High-resolution structure of the human GPR40 receptor bound to allosteric agonist TAK-875. Nature 513, 124–127 (2014).
Hirozane, Y., Motoyaji, T., Maru, T., Okada, K. & Tarui, N. Generating thermostabilized agonist-bound GPR40/FFAR1 using virus-like particles and a label-free binding assay. Mol. Membr. Biol. 31, 168–175 (2014).
Jazayeri, A. et al. Extra-helical binding site of a glucagon receptor antagonist. Nature 533, 274–277 (2016).
Zhang, D. et al. Two disparate ligand-binding sites in the human P2Y1 receptor. Nature 520, 317–321 (2015).
Kruse, A.C. et al. Activation and allosteric modulation of a muscarinic acetylcholine receptor. Nature 504, 101–106 (2013).
Hauge, M. et al. GPR40 (FFAR1)—combined Gs and Gq signaling in vitro is associated with robust incretin secretagogue action ex vivo and in vivo. Mol. Metab. 4, 3–14 (2014).
Lee, A.G. Lipid-protein interactions in biological membranes: a structural perspective. Biochim. Biophys. Acta 1612, 1–40 (2003).
Vinothkumar, K.R. Structure of rhomboid protease in a lipid environment. J. Mol. Biol. 407, 232–247 (2011).
Sum, C.S. et al. Identification of residues important for agonist recognition and activation in GPR40. J. Biol. Chem. 282, 29248–29255 (2007).
Tikhonova, I.G. & Poerio, E. Free fatty acid receptors: structural models and elucidation of ligand binding interactions. BMC Struct. Biol. 15, 16 (2015).
Rasmussen, S.G. et al. Crystal structure of the β2 adrenergic receptor-Gs protein complex. Nature 477, 549–555 (2011).
Kang, Y. et al. Crystal structure of rhodopsin bound to arrestin by femtosecond X-ray laser. Nature 523, 561–567 (2015).
Carpenter, B., Nehmé, R., Warne, T., Leslie, A.G. & Tate, C.G. Structure of the adenosine A(2A) receptor bound to an engineered G protein. Nature 536, 104–107 (2016).
Huang, W. et al. Structural insights into μ-opioid receptor activation. Nature 524, 315–321 (2015).
Katritch, V., Cherezov, V. & Stevens, R.C. Structure-function of the G protein-coupled receptor superfamily. Annu. Rev. Pharmacol. Toxicol. 53, 531–556 (2013).
Wacker, D. et al. Structural features for functional selectivity at serotonin receptors. Science 340, 615–619 (2013).
Wang, C. et al. Structural basis for molecular recognition at serotonin receptors. Science 340, 610–614 (2013).
Venkatakrishnan, A.J. et al. Molecular signatures of G-protein-coupled receptors. Nature 494, 185–194 (2013).
Moro, O., Lameh, J., Högger, P. & Sadée, W. Hydrophobic amino acid in the i2 loop plays a key role in receptor-G protein coupling. J. Biol. Chem. 268, 22273–22276 (1993).
Burstein, E.S., Spalding, T.A. & Brann, M.R. The second intracellular loop of the m5 muscarinic receptor is the switch which enables G-protein coupling. J. Biol. Chem. 273, 24322–24327 (1998).
Warne, T. et al. Structure of a beta1-adrenergic G-protein-coupled receptor. Nature 454, 486–491 (2008).
Jaakola, V.P. et al. The 2.6 angstrom crystal structure of a human A2A adenosine receptor bound to an antagonist. Science 322, 1211–1217 (2008).
Perez-Aguilar, J.M., Shan, J., LeVine, M.V., Khelashvili, G. & Weinstein, H. A functional selectivity mechanism at the serotonin-2A GPCR involves ligand-dependent conformations of intracellular loop 2. J. Am. Chem. Soc. 136, 16044–16054 (2014).
Kobilka, B. The structural basis of G-protein-coupled receptor signaling (Nobel Lecture). Angew. Chem. Int. Edn Engl. 52, 6380–6388 (2013).
Cooke, R. & Congreve, M. Allosteric binding: structures reveal new ways to tame G protein-coupled receptors. Future Med. Chem. 8, 2007–2007 (2016).
Molecular Operating Environment (MOE) 2013.08 (Chemical Computing Group ULC, 2017).
Kenakin, T. Allosteric agonist modulators. J. Recept. Signal Transduct. Res. 27, 247–259 (2007).
Ehlert, F.J. Estimation of the affinities of allosteric ligands using radioligand binding and pharmacological null methods. Mol. Pharmacol. 33, 187–194 (1988).
Caffrey, M. & Cherezov, V. Crystallizing membrane proteins using lipidic mesophases. Nat. Protoc. 4, 706–731 (2009).
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997).
Kabsch, W. Xds. Acta Crystallogr. D Biol. Crystallogr. 66, 125–132 (2010).
Vonrhein, C. et al. Data processing and analysis with the autoPROC toolbox. Acta Crystallogr. D Biol. Crystallogr. 67, 293–302 (2011).
Winn, M.D. et al. Overview of the CCP4 suite and current developments. Acta Crystallogr. D Biol. Crystallogr. 67, 235–242 (2011).
McCoy, A.J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Murshudov, G.N. et al. REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr. D Biol. Crystallogr. 67, 355–367 (2011).
BUSTER v. 2.11.6 (Global Phasing Ltd., 2016).
Chen, V.B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 66, 12–21 (2010).
The PyMOL Molecular Graphics System v. 1.8 (Schrödinger, LLC).
Jones, G., Willett, P., Glen, R.C., Leach, A.R. & Taylor, R. Development and validation of a genetic algorithm for flexible docking. J. Mol. Biol. 267, 727–748 (1997).
Acknowledgements
This manuscript is dedicated to the late Frank K. Brown for his groundbreaking contributions to drug discovery and his vision and tenacity in making this research possible. We also thank K. Hollenstein and H. Krishnamurthy for helpful discussions and technical insights. This research used resources at the Industrial Macromolecular Crystallography Association Collaborative Access Team (IMCA-CAT) beamline 17-ID, supported by the companies of the Industrial Macromolecular Crystallography Association through a contract with Hauptman-Woodward Medical Research Institute. We thank K. Battaile and A. M. Mulichak for data collection assistance and L. J. Keefe for direction of IMCA-CAT operations. This research was performed at the Advanced Photon Source, a US Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
Author information
Authors and Affiliations
Contributions
S.M.S., S. Sharma, A.B.W., J.M.J., and S.J.A. conceived and managed the project with support from F.K.B., M.W., A.D.H., K.J.L. and J.D.H. N.B., S.J.A., D.L.H., J.H., T.H., M.K., H.W., A.B.W., P.S. and S.T. designed, optimized, purified and characterized receptor constructs for structural studies. J.L. and S.B.P. crystallized the receptor in LCP. J.L. supervised data collection and J.L., C.V. and G.B. processed synchrotron data. J.L. solved and refined the structures. J.L., J.M.J., J.W., A.B.W., J.D.S., S.M.S. and S. Sharma interpreted the structure and designed experiments. J.W., J.H. and D.L.H. prepared cells for binding studies. J.W., J.D.S. and A.B.W. designed the binding experiments. J.W. carried out radioligand-binding assays with cells. B.T.F., A.B.W., N.B.H. and S. Souza conceived and performed cell-based functional assays. J.L., N.B., J.W., A.B.W., J.M.J., S. Sharma, and S.M.S. analyzed the data and compiled the figures for the manuscript. J.M.J. and B.S.S. designed and performed docking and molecular modeling. H.R.C., S.L.C., Y.G., H.J., M.W.M., B.P., and C.W.P. selected and synthesized compounds for pharmacological profiling, receptor purification, and crystallization studies. J.L., N.B. and J.W. wrote the manuscript with contributions from J.M.J., A.B.W., S. Sharma and S.M.S.
Corresponding authors
Ethics declarations
Competing interests
All authors, with the exception of G.B. and C.V., are current or past employees of Merck Research Laboratories.
Integrated supplementary information
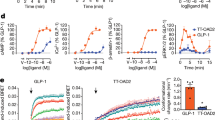
Supplementary Figure 1 Activation of GPR40 by select synthetic ligands.
(a) Chemical structures of the synthetic agonists used in this study. Partial agonists [TAK875, MK-8666 & P4] and AgoPAMs [AP4 & AP8] chemical structures. (b) Dose-response curves for the five agonists shown in (a) were generated monitoring IP accumulation in HEK293 cells expressing human GPR40. Data are expressed as a percentage of the control response of an in-house partial agonist, and fitted to a standard 4-parameter non-linear regression model. EC50’s were determined for each test compound using a custom in-house developed software package. Each agonist EC50 and Emax was calculated at least six times (mean parameters ± SD in Supplementary Table 1) with representative curves (n=1) illustrated here.
Supplementary Figure 2 Saturation binding of [3H]P4 or [3H]AP8 to hGPR40 variants.
Panels show [3H]P4 or [3H]AP8 total (black circle), non-specific (open black circle) and specific (red circle) binding to the WT receptor (a & b) or the triple mutant (c & d) with a T4L insertion. Each study was repeated twice (N=2), with symbols in the representative graphs shown denoting the mean values of a single experiment ± SD (n=2). The mean parameters of these and other individual experiments are shown in Supplementary Table 3.
Supplementary Figure 3 GPR40 purification, LCP crystallization drop image and the electron density map for the AgoPAM AP8
(a) Schematic of GPR40 purification. Purification begins from isolated membranes from insect cells. (b) Final SEC trace shows that only select fractions of >95% purity were selected for the final pool. This pool corresponds to the monomeric form of GPR40. Purity is observed in the SDS-Page gel shown below the chromatogram. (c) Crystals obtained in lipidic cubic phase viewed under (Left) regular light and (Right) circularly polarized light. (d) The stereo image of the omit maps for AP8 in the ternary complex (3.5σ).
Supplementary Figure 4 Snake-plot diagram of the GPR40 construct and summary of AgoPAM AP8 interactions.
(a) Snake-plot diagram of the GPR40 construct(s) in crystallization. Thermostabilizing mutations are shown in red, Cysteines forming disulfide bond are shown in yellow, the affinity tags are shown in light blue, most conserved residues for each TM are colored in dark gray, residues interacting with MK-8666 are colored in green and residues interacting with AP8 are in gold. Note Tyr913.37 is colored half green and half gold since it is the only amino acid making direct interactions with both MK-8666 and AP8. (b) AgoPAM AP8 interactions with GPR40 using Molecular Operating Environment (MOE)46. Schematic representation of the interactions between AP8 and the AgoPAM binding site following the MOE standard structure preparation and 3D (rule-based) protonation protocols.
Supplementary Figure 5 Cell based [3H]AP8 and [3H]P4 saturation binding to hGPR40 WT receptor and binding-pocket mutants.
[3H]AP8 (a-b) or [3H]P4 (c-d) binding to WT (black circle), Y44F (red circle), Y114F (green circle), S123A (blue circle), G95F (yellow circle), A99L (purple circle) and Al02W (cyan circle). Each study was repeated twice (N=2), with symbols in the representative graphs shown denoting the mean values of a single experiment ± SD (n=2). KD values are displayed in Table 2. Data is transformed with a log plot of x-axis to illustrate effects on KD (b & d).
Supplementary Figure 6 Identification & application of [3H]AP4 for interrogation of the AgoPAM binding site in dog GPR40.
(a-f) [3H]AP4 binds with high affinity to dog GPR40. Saturation binding of [3H]P4 (a & d, 1.5 & 2.4 nM respectively), [3H]AP8 (b & e, 1.6 & >125 nM respectively) or [3H]AP4 (c & f, 1.3 & 12.8 nM respectively) to human (a-c) or dog (d-f) GPR40. Total (black circle), non-specific (open black circle) and specific (red circle) binding are denoted as described. Each experiment was repeated twice with representative graphs (n=2) shown. Notably, while [3H]AP8 is a suitable tool to profile AgoPAM binding to human GPR40, weaker observed affinity for dog GPR40 dictated replacement with the higher affinity tool, [3H]AP4. (g-j) AgoPAM affinity can be modulated in human and dog GPR40 by mutagenesis of position 102. Human GPR40 [3H]AP8 binding affinity is reduced by replacing Ala102 with larger side chains (g) while [3H]P4 KD (h) is not significantly changed [human GPR40 WT (black circle), A102V (red circle), A102I (green circle) and A102W (blue circle)]. Dog GPR40 [3H]AP4 binding affinity is modulated bi-directionally by varying the bulk of the side chain at Val102 (i) while [3H]P4 KD (j) is not significantly changed [dog GPR40 WT (black circle), V102A (red circle), V102I (green circle) and V102W (blue circle)]. Each study was repeated twice (N=2), with symbols in the representative graphs shown denoting the mean values of a single experiment ± SD (n=2). KD values are displayed in Supplementary Table 6. Data is transformed with a log plot of x-axis to illustrate effects on KD.
Supplementary Figure 7 Examination of binding and cooperativity in the hGPR40 quadruple mutant with a T4L insertion.
(a-b) Saturation binding of [3H]P4 or [3H]AP8 to Quad hGPR40. [3H]P4 (a) or [3H]AP8 (b) total (black circle), non-specific (open black circle) and specific (red circle) binding to the quadruple mutant with a T4L insertion. (c-d) MK-8666 binds with high affinity to displace [3H]P4 from hGPR40 quadruple mutant-T4L (red circle), while augmenting the binding of [3H]AP8 to the same receptor (red circle). In a reciprocal manner, AP8 displaces [3H]AP8 from the hGPR40 quadruple mutant-T4L (black circle), while augmenting the binding of [3H]P4 to the same receptors (black circle). Each study was repeated twice (N=2), with symbols in the representative graphs shown denoting the mean values of a single experiment ± SD (n=2).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 and Supplementary Tables 1–8. (PDF 2191 kb)
Conformational changes between GPR40 binary complex and ternary complex upon the binding of the AgoPAM AP8.
A video showing the conformation changes in hGPR40 from the binary complex with MK-8666 to the ternary complex with both MK-8666 and AP8, made using a morphing technique. (MPG 8328 kb)
Rights and permissions
About this article
Cite this article
Lu, J., Byrne, N., Wang, J. et al. Structural basis for the cooperative allosteric activation of the free fatty acid receptor GPR40. Nat Struct Mol Biol 24, 570–577 (2017). https://doi.org/10.1038/nsmb.3417
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.3417
This article is cited by
-
G protein-coupled receptors (GPCRs): advances in structures, mechanisms, and drug discovery
Signal Transduction and Targeted Therapy (2024)
-
Cryo-electron microscopy for GPCR research and drug discovery in endocrinology and metabolism
Nature Reviews Endocrinology (2024)
-
Structure of GPR101–Gs enables identification of ligands with rejuvenating potential
Nature Chemical Biology (2024)
-
Molecular recognition and activation mechanism of short-chain fatty acid receptors FFAR2/3
Cell Research (2024)
-
Ligand recognition and allosteric modulation of the human MRGPRX1 receptor
Nature Chemical Biology (2023)