Abstract
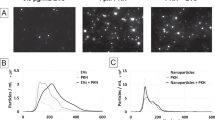
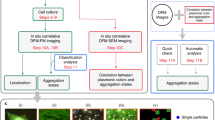
Nanoparticle size, surface charge and material composition are known to affect the uptake of nanoparticles by cells. However, whether nanoparticle shape affects transport across various barriers inside the cell remains unclear. Here we used pair correlation microscopy to show that polymeric nanoparticles with different shapes but identical surface chemistries moved across the various cellular barriers at different rates, ultimately defining the site of drug release. We measured how micelles, vesicles, rods and worms entered the cell and whether they escaped from the endosomal system and had access to the nucleus via the nuclear pore complex. Rods and worms, but not micelles and vesicles, entered the nucleus by passive diffusion. Improving nuclear access, for example with a nuclear localization signal, resulted in more doxorubicin release inside the nucleus and correlated with greater cytotoxicity. Our results therefore demonstrate that drug delivery across the major cellular barrier, the nuclear envelope, is important for doxorubicin efficiency and can be achieved with appropriately shaped nanoparticles.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
30 September 2016
In the version of this Article originally published online, in the Methods, in the equation for G(τ), in the denominator, the superscript '2' was incorrectly placed, and in the equation for G(τ, δr), in the denominator, there should have been two sets of angled brackets. These errors have been corrected in all versions of the Article.
References
Jiang, W., Kim, B. Y., Rutka, J. T. & Chan, W. C. Nanoparticle-mediated cellular response is size-dependent. Nat. Nanotechnol. 3, 145–150 (2008).
Davis, M. E., Chen, Z. G. & Shin, D. M. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat. Rev. Drug. Discov. 7, 771–782 (2008).
Ahn, S., Seo, E., Kim, K. & Lee, S. J. Controlled cellular uptake and drug efficacy of nanotherapeutics. Sci. Rep. 3, 1997 (2013).
Ruthardt, N., Lamb, D. C. & Brauchle, C. Single-particle tracking as a quantitative microscopy-based approach to unravel cell entry mechanisms of viruses and pharmaceutical nanoparticles. Mol. Ther. 19, 1199–1211 (2011).
Yarbrough, M. L., Mata, M. A., Sakthivel, R. & Fontoura, B. M. Viral subversion of nucleocytoplasmic trafficking. Traffic 15, 127–140 (2014).
Dohner, K. & Sodeik, B. The role of the cytoskeleton during viral infection. Curr. Top. Microbiol. Immunol. 285, 67–108 (2005).
Champion, J. A., Katare, Y. K. & Mitragotri, S. Making polymeric micro- and nanoparticles of complex shapes. Proc. Natl Acad. Sci. USA 104, 11901–11904 (2007).
Champion, J. A., Katare, Y. K. & Mitragotri, S. Particle shape: a new design parameter for micro- and nanoscale drug delivery carriers. J. Control Release 121, 3–9 (2007).
Karagoz, B., Boyer, C. & Davis, T. P. Simultaneous polymerization-induced self-assembly (PISA) and guest molecule encapsulation. Macromol. Rapid Commun. 35, 417–421 (2014).
Barua, S. et al. Particle shape enhances specificity of antibody-displaying nanoparticles. Proc. Natl Acad. Sci. USA 110, 3270–3275 (2013).
Chu, Z. et al. Unambiguous observation of shape effects on cellular fate of nanoparticles. Sci. Rep. 4, 4495 (2014).
Chithrani, B. D. & Chan, W. C. Elucidating the mechanism of cellular uptake and removal of protein-coated gold nanoparticles of different sizes and shapes. Nano. Lett. 7, 1542–1550 (2007).
Agarwal, R. et al. Mammalian cells preferentially internalize hydrogel nanodiscs over nanorods and use shape-specific uptake mechanisms. Proc. Natl Acad. Sci. USA 110, 17247–17252 (2013).
Xu, Z. P. et al. Subcellular compartment targeting of layered double hydroxide nanoparticles. J. Control Release 130, 86–94 (2008).
Hinde, E., Cardarelli, F., Digman, M. A. & Gratton, E. In vivo pair correlation analysis of EGFP intranuclear diffusion reveals DNA-dependent molecular flow. Proc. Natl Acad. Sci. USA 107, 16560–16565 (2010).
Hinde, E., Cardarelli, F., Digman, M. A. & Gratton, E. Changes in chromatin compaction during the cell cycle revealed by micrometer-scale measurement of molecular flow in the nucleus. Biophys. J. 102, 691–697 (2012).
Hinde, E. et al. The impact of mitotic versus interphase chromatin architecture on the molecular flow of EGFP by pair correlation analysis. Biophys. J. 100, 1829–1836 (2011).
Hinde, E., Kong, X., Yokomori, K. & Gratton, E. Chromatin dynamics during DNA repair revealed by pair correlation analysis of molecular flow in the nucleus. Biophys. J. 107, 55–65 (2014).
Digman, M. A. & Gratton, E. Imaging barriers to diffusion by pair correlation functions. Biophys. J. 97, 665–673 (2009).
Digman, M. A. & Gratton, E. Lessons in fluctuation correlation spectroscopy. Annu. Rev. Phys. Chem. 62, 645–668 (2011).
dos Santos, T., Varela, J., Lynch, I., Salvati, A. & Dawson, K. A. Effects of transport inhibitors on the cellular uptake of carboxylated polystyrene nanoparticles in different cell lines. PLoS ONE 6, e24438 (2011).
Chu, Z. et al. Rapid endosomal escape of prickly nanodiamonds: implications for gene delivery. Sci. Rep. 5, 11661 (2015).
Cardarelli, F. & Gratton, E. In vivo imaging of single-molecule translocation through nuclear pore complexes by pair correlation functions. PLoS ONE 5, e10475 (2010).
Scott, C. C., Vacca, F. & Gruenberg, J. Endosome maturation, transport and functions. Semin. Cell Dev. Biol. 31, 2–10 (2014).
Huotari, J. & Helenius, A. Endosome maturation. EMBO J. 30, 3481–3500 (2011).
Kerr, M. & Teasdale, R. D. Live imaging of endosome dynamics. Semin. Cell. Dev. Biol. 31, 11–19 (2014).
Alber, F. et al. The molecular architecture of the nuclear pore complex. Nature 450, 695–701 (2007).
Adams, R. L. & Wente, S. R. Uncovering nuclear pore complexity with innovation. Cell 152, 1218–1221 (2013).
Ghosh, K., Kanapathipillai, M., Korin, N., McCarthy, J. R. & Ingber, D. E. Polymeric nanomaterials for islet targeting and immunotherapeutic delivery. Nano Letters 12, 203–208 (2012).
Misra, R. & Sahoo, S. K. Intracellular trafficking of nuclear localization signal conjugated nanoparticles for cancer therapy. Eur. J. Pharm. Sci. 39, 152–163 (2010).
Karagoz, B. et al. Polymerization-induced self-assembly (PISA) - control over the morphology of nanoparticles for drug delivery applications. Poly. Chem. 5, 350–355 (2014).
Acknowledgements
E.H. is funded by a Cancer Institute NSW Early Career Fellowship (RG151879) and UNSW Vice Chancellor Research Fellowship. B.K. acknowledges the Scientific and Technological Research Council of Turkey (TUBITAK) for financial support. C.B. is funded by a Future Fellowship (FT1210096) from Australian Research Council (ARC). K.G. acknowledges funding from the ARC Centre of Excellence in Advanced Molecular Imaging (CE140100011) and National Health and Medical Research Council of Australia (1059278, 1037320). J.J.G. acknowledges funding from the ARC Centre of Excellence in Convergent Bio-Nano Science and Technology (CE140100036), the ARC Laureate Fellowship (FL150100060) program and a National Health and Medical Research Council program grant (1091261). The authors thank C. Benzing (UNSW) for discussion on cell uptake and endosomal escape. The authors thank E. Gratton (University of California, Irvine) for discussion on data analysis. The work was supported by the BioMedical Imaging Facility at UNSW.
Author information
Authors and Affiliations
Contributions
E.H, C.B, J.J.G and K.G designed the research. E.H and K.T performed imaging experiments. H.T.T.D, B.K and J.Y synthesized the nanoparticles. E.H and K.T. analysed data, and E.H., J.J.G and K.G. wrote the paper. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 1725 kb)
Rights and permissions
About this article
Cite this article
Hinde, E., Thammasiraphop, K., Duong, H. et al. Pair correlation microscopy reveals the role of nanoparticle shape in intracellular transport and site of drug release. Nature Nanotech 12, 81–89 (2017). https://doi.org/10.1038/nnano.2016.160
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2016.160
This article is cited by
-
Molecular Mechanisms of Intracellular Delivery of Nanoparticles Monitored by an Enzyme-Induced Proximity Labeling
Nano-Micro Letters (2024)
-
Nanovaccine-based strategies for lymph node targeted delivery and imaging in tumor immunotherapy
Journal of Nanobiotechnology (2023)
-
Heterogeneity of mesenchymal stem cell-derived extracellular vesicles is highly impacted by the tissue/cell source and culture conditions
Cell & Bioscience (2022)
-
Inhibition of the CEBPβ-NFκB interaction by nanocarrier-packaged Carnosic acid ameliorates glia-mediated neuroinflammation and improves cognitive function in an Alzheimer’s disease model
Cell Death & Disease (2022)
-
Organelle-targeted therapies: a comprehensive review on system design for enabling precision oncology
Signal Transduction and Targeted Therapy (2022)