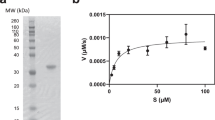
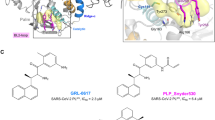
Abstract
Designing selective inhibitors of proteases has proven problematic, in part because pharmacophores that confer potency exploit the conserved catalytic apparatus. We developed a fundamentally different approach by designing irreversible inhibitors that target noncatalytic cysteines that are structurally unique to a target in a protein family. We have successfully applied this approach to the important therapeutic target HCV protease, which has broad implications for the design of other selective protease inhibitors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Accession codes
References
Turk, B. Nat. Rev. Drug Discov. 5, 785–799 (2006).
Puente, X.S., Sanchez, L.M., Overall, C.M. & Lopez-Otin, C. Nat. Rev. Genet. 4, 544–558 (2003).
Smith, A.J., Zhang, X., Leach, A.G. & Houk, K.N. J. Med. Chem. 52, 225–233 (2009).
Robertson, J.G. Biochemistry 44, 5561–5571 (2005).
Bartenschlager, R. & Sparacio, S. Virus Res. 127, 195–207 (2007).
Perni, R.B. et al. Antimicrob. Agents Chemother. 50, 899–909 (2006).
Lin, C., Kwong, A.D. & Perni, R.B. Infect. Disord. Drug Targets 6, 3–16 (2006).
McHutchison, J.G. et al. N. Engl. J. Med. 360, 1827–1838 (2009).
Stauber, R.E. & Kessler, H.H. Drugs 68, 1347–1359 (2008).
Njoroge, F.G., Chen, K.X., Shih, N.Y. & Piwinski, J.J. Acc. Chem. Res. 41, 50–59 (2008).
Venkatraman, S. et al. J. Med. Chem. 49, 6074–6086 (2006).
Lamarre, D. et al. Nature 426, 186–189 (2003).
Lin, T.I. et al. Antimicrob. Agents Chemother. 53, 1377–1385 (2009).
Seiwert, S.D. et al. Antimicrob. Agents Chemother. 52, 4432–4441 (2008).
McCauley, J.A. et al. J. Med. Chem. 53, 2443–2463 (2010).
Liverton, N.J. et al. Antimicrob. Agents Chemother. 54, 305–311 (2010).
Ling, C. in Hepatitis C Viruses: Genomes and Molecular Biology. (ed. Tan, S.-L.) 163–206 (Horizon Biosciences, 2006).
Campbell, J.A. & Good, A.C. World Intellectual Property Organization Patent, WO 2003053349 A2 (2003).
Thompson, A.J. & McHutchison, J.G. J. Viral Hepat. 16, 377–387 (2009).
Kou, Y.H., Chang, M.F., Wang, Y.M., Hung, T.M. & Chang, S.C. J. Virol. 81, 7999–8008 (2007).
Singh, J., Petter, R.C. & Kluge, A.F. Curr. Opin. Chem. Biol. 14, 475–480 (2010).
Potashman, M.H. & Duggan, M.E. J. Med. Chem. 52, 1231–1246 (2009).
Hagel, M. et al. Reviews in Antiviral Therapy & Infectious Diseases 5, 15 (2009).
Acknowledgements
We thank the following for critical reading of the manuscript: A. Whitty, B. Lindenbach, S. Witowski and N. Mahanthappa. We also thank R. Bartenschlager for helpful conversations. We thank Proteros Biostructures for help with X-ray crystallographic studies and A. Prasad for help with figures.
Author information
Authors and Affiliations
Contributions
M.H., T.S.M., M.P.S., M.T.L., H.B., R.M.K. and P.C. performed experiments; D.N., L.Q., J.S. and R.C.P. designed and generated chemical compounds; Z.Z. and J.S. performed molecular modeling; M.N., W.F.W., R.C.P. and J.S. supervised the project; M.H., M.N., R.C.P. and J.S. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
All authors are employees and shareholders of Avila Therapeutics, except H.B. who is a former employee.
Supplementary information
Supplementary Text and Figures
Supplementary Methods, Supplementary Schemes 1–4, Supplementary Figures 1–11 and Supplementary Tables 1–3 (PDF 1372 kb)
Rights and permissions
About this article
Cite this article
Hagel, M., Niu, D., St Martin, T. et al. Selective irreversible inhibition of a protease by targeting a noncatalytic cysteine. Nat Chem Biol 7, 22–24 (2011). https://doi.org/10.1038/nchembio.492
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.492
This article is cited by
-
Delving into the Characteristic Features of “Menace” Mycobacterium tuberculosis Homologs: A Structural Dynamics and Proteomics Perspectives
The Protein Journal (2020)
-
Covalent Versus Non-covalent Enzyme Inhibition: Which Route Should We Take? A Justification of the Good and Bad from Molecular Modelling Perspective
The Protein Journal (2020)
-
Road Map for the Structure-Based Design of Selective Covalent HCV NS3/4A Protease Inhibitors
The Protein Journal (2017)
-
The pharmacological landscape and therapeutic potential of serine hydrolases
Nature Reviews Drug Discovery (2012)
-
New approaches for dissecting protease functions to improve probe development and drug discovery
Nature Structural & Molecular Biology (2012)