Abstract
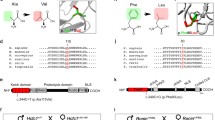
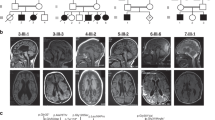
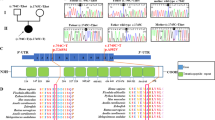
Neurospora crassa ARG13 and Saccharomyces cerevisiae ARG11 encode mitochondrial carrier family (MCF) proteins that transport ornithine across the mitochondrial inner membrane. We used their sequences to identify EST candidates that partially encode orthologous mammalian transporters. We thereby identified such a gene (ORNT1) that maps to 13q14 and whose expression, similar to that of other urea cycle (UC) components, was high in liver and varied with changes in dietary protein. ORNT1 expression restores ornithine metabolism in fibroblasts from patients with hyperammonaemia-hyperornithinaemia -homocitrullinuria (HHH) syndrome. In a survey of 11 HHH probands, we identified 3 ORNT1 mutant alleles that account for 21 of 22 possible mutant ORNT1 genes in our patients: F188Δ, which is common in French-Canadian HHH patients and encodes an unstable protein; E180K, which encodes a stable, properly targeted protein that is inactive; and a 13q14 microdeletion. Our results show that ORNT1 encodes the mitochondrial ornithine transporter involved in UC function and is defective in HHH syndrome.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Valle, D. & Simell, O. The hyperornithinemias. in The Metabolic and Molecular Bases of Inherited Disease (eds Scriver, C., Beaudet, A., Sly, W. & Valle, D.) 1147–1185 (McGraw Hill, New York, 1995).
Brusilow, S.W. & Horwich, A.L. Urea cycle enzymes. in The Metabolic and Molecular Bases of Inherited Disease (eds Scriver, C., Beaudet, A., Sly, W. & Valle, D.) 1187–1232 (McGraw Hill, New York, 1995).
Gamble, G. & Lehninger, A.L. Transport of ornithine and citrulline across the mitochondrial membrane. J. Biol. Chem. 248 , 610–618 (1973).
Bradford, N.M. & McGivan, J.D. Evidence for the existence of an ornithine/citrulline antiporter in rat liver mitochondria. FEBS Lett. 113, 294–298 (1980).
Indiveri, C., Tonazzi, A. & Palmieri, F. Identification and purification of the ornithine/citrulline carrier from rat liver mitochondria. Eur. J. Biochem. 207, 449–454 (1992).
Indiveri, C., Tonazzi, A., Stipani, I. & Palmieri, F. The purified and reconstituted ornithine/citrulline carrier from rat liver mitochondria: electrical nature and coupling of the exchange reaction with H+ translocation. Biochem. J. 327, 349– 356 (1997).
Indiveri, C., Palmieri, L. & Palmieri, F. Kinetic characterization of the reconstituted ornithine carrier from rat liver mitochondria. Biochim. Biophys. Acta 1188, 293–301 (1994).
Shih, V., Efron, M.L. & Moser, H.W. Hyperornithinemia, hyperammonemia, and homocitrullinuria. A new disorder of amino acid metabolism associated with myoclonic seizures and mental retardation. Am. J. Dis. Child. 117, 83–92 (1969).
Fell, V., Pollitt, R.J., Sampson, G.A. & Wright, T. Ornithinemia, hyperammonemia, and homocitrullinuria. A disease associated with mental retardation and possibly caused by defective mitochondrial transport. Am. J. Dis. Child. 127, 752– 756 (1974).
Lemay, J. et al. HHH syndrome: neurologic, ophthalmologic and psychological evaluation of six patients. J. Pediatr. 121, 725– 730 (1992).
Shih, V.E., La Framboise, R., Mandell, R. & Pichette, J. Neonatal form of the hyperornithinaemia, hyperammonaemia and homocitrullinuria (HHH) syndrome and prenatal diagnosis. Prenat. Diagn. 12, 717–723 (1992).
Zammarchi, E. et al. Neonatal onset of hyperornithinemia-hyperammonemia- homocitrullinuria syndrome with favorable outcome. J. Pediatr. 131, 440–443 (1997).
Palmieri, F., Indiveri, C., Bisaccia, F. & Iacobazzi, V. Mitochondrial metabolite carrier proteins: purification, reconstitution and transport studies. Methods Enzymol. 260, 349–369 (1995).
Palmieri, F. Mitochondrial carrier protein. FEBS Lett. 346, 48–54 (1994).
Moualij, B., Duyckaerts, C., Lamotte-Brasseur, J. & Sluse, F.E. Phylogenetic classification of the mitochondrial carrier family of Saccharomyces cerevisiae. Yeast 13, 573– 581 (1997).
Nelson, D.R., Felix, C.M. & Swanson, J.M. Highly conserved charge-pair networks in the mitochondrial carrier family. J. Mol. Biol. 277, 285– 308 (1998).
Krämer, R. Structural and functional aspects of the phosphate carrier from mitochondria. Kidney Int. 49, 947–952 (1996).
Schroers, A., Burkovski, A., Wohlrab, H. & Krämer, R. The phosphate carrier from yeast mitochondria. J. Biol. Chem. 273, 14269–14276 (1998).
Crabeel, M., Soetens, O., De Rijcke, M., Pratiwi, R. & Pankiewicz, R. The ARG11 gene of Saccharomyces cerevisiae encodes a mitochondrial integral membrane protein required for arginine biosynthesis. J. Biol. Chem. 271, 25011–25018 (1996).
Palmieri, L. et al. Identification of the yeast ARG-11 gene as a mitochondrial ornithine carrier involved in arginine biosynthesis. FEBS Lett. 410, 447–451 ( 1997).
Liu, Q. & Dunlap, J.C. Isolation and analysis of the arg-113 gene of Neurospora crassa. Genetics 143, 1163–1174 (1996).
Braverman, N. et al. Human PEX7 encodes the peroxisomal PTS2 receptor and is responsible for rhizomelic chondrodysplasia punctata. Nature Genet. 15, 369–376 ( 1997).
Indiveri, C., Iacobazzi, V., Giangregorio, N. & Palmieri, F. Bacterial overexpression, purification and reconstitution of the carnitine/acylcarnitine carrier from rat liver mitochondria. Biochem. Biophys. Res. Commun. 249, 589–594 ( 1998).
Huizing, M. et al. Cloning of the human carnitine acylcarnitine carrier cDNA and identification of the molecular defect in a patient. Am. J. Hum. Genet. 61, 1239–1245 (1997).
Morris, S.M.J. et al. Regulation of mRNA levels for five urea cycle enzymes in rat liver by diet, cyclic AMP, and glucocorticoids. Arch. Biochem. Biophys. 256, 343–353 ( 1987).
Schimke, R.T. Adaptive characteristics of urea cycle enzymes in the rat. J. Biol. Chem. 237, 459–463 ( 1962).
Kozak, M. An analysis of vertebrate mRNA sequences: intimations of translational control. J. Cell Biol. 115, 887– 903 (1991).
Cayanis, E. et al. High resolution YAC-cosmid-STS map of human chromosome 13. Genomics 47, 26–43 (1998).
Shih, V.E., Mandell, R. & Herzfeld, A. Defective ornithine metabolism in cultured skin fibroblasts from patients with the syndrome of hyperornithinemia, hyperammonemia and homocitrullinuria. Clin. Chim. Acta 118, 149– 157 (1982).
Weber, F.E. et al. Molecular cloning of a peroxisomal Ca2+-dependent member of the mitochondrial carrier superfamily. Proc. Natl Acad. Sci. USA 94, 8509–8514 ( 1997).
Wylin, T. et al. Identification and characterization of human PMP34, a protein closely related to the peroxisomal integral membrane protein PMP47 of Candida boidinii. Eur. J. Biochem. 258, 332–338 (1998).
Dionisi Vici, C., Bachmann, C., Gambarara, M., Colombo, J.P. & Sabetta, G. Hyperornithinemia-hyperammonemia- homocitrullinuria syndrome: low creatine excretion and effect of citrulline, arginine, or ornithine supplement. Pediatr. Res. 22, 364– 367 (1987).
Gatfield, P.D., Taller, E., Wolfe, D.M. & Haust, D.M. Hyperornithinemia, hyperammonemia, and homocitrullinuria associated with decreased carbamyl phosphate synthetase I activity. Pediatr. Res. 9, 488–497 (1975).
Simell, O., Mackenzie, S., Clow, C.L. & Scriver, C.R. Ornithine loading did not prevent induced hyperammonemia in a patient with HHH syndrome. Pediatr. Res. 19, 1283– 1287 (1985).
Smith, L. et al. Hyperornithinemia, hyperammonemia, homocitrullinuria (HHH) syndrome: presentation as acute liver disease with coagulopathy. J. Pediatr. Gastroenterol. Nutr. 15, 431–436 (1992).
Mitchell, G.A., Grompe, M., Lambert, M. & Tanguay, R. Hypertyrosinemia. in The Metabolic and Molecular Bases of Inherited Disease (eds Scriver, C., Beaudet, A., Sly, W. & Valle, D.) (McGraw-Hill, New York, in press).
De Braekeleer, M. et al. Founder effect in familial hyperchylomicronemia among French Canadians of Quebec. Hum. Hered. 41, 168 –173 (1991).
Bouchard, J.P. et al. Autosomal recessive spastic ataxia of Charlevoix-Saguenay. Neuromuscul. Disord. 8, 474– 479 (1998).
Morin, C. et al. Clinical, metabolic and genetic aspects of cytochrome C oxidase deficiency in Saguenay-Lac-Saint-Jean. Am. J. Hum. Genet. 52, 488–496 (1993).
Merante, F. et al. A biochemically distinct form of cytochrome oxidase (COX) deficiency in the Saguenay-Lac-Saint-Jean region of Quebec. Am. J. Hum. Genet. 53, 481–487 (1993).
Charbonneau, H. et al. Naissance d'une Population. Les Français établis au Canada au XVIIe siécle (Presses de l'Université de Montréal, Montreal, 1987).
Carter, K.C. et al. Mutation at the phenylalanine hydroxylase gene (PAH) and its use to document population genetic variation: the Quebec experience. Eur. J. Hum. Genet. 6, 61–70 (1998).
Vohl, M.C. et al. Geographic distribution of French-Canadian low density lipoprotein receptor gene mutations in the Province of Quebec. Clin. Genet. 52, 1–6 (1997 ).
Brais, B. et al. Short GCG expansions in the PABP2 gene cause oculopharyngeal muscular dystrophy. Nature Genet. 18, 164 –167 (1998).
Passarella, S., Atlante, A. & Quagliariello, E. Ornithine/phosphate antiport in rat kidney mitochondria. Eur. J. Biochem. 193, 221– 227 (1990).
Indiveri, C., Iacobazzi, V., Giangregorio, N. & Palmieri, F. The mitochondrial carnitine carrier protein: cDNA cloning, primary structure and comparison with other mitochondrial transport proteins. Biochem. J. 321, 713–719 ( 1997).
Haust, M.D., Dewar, R.A., Gatfield, D.P. & Gordon, B.A. Hyperornithemia-hyperammonemia- homocitrullinuria (HHH) syndrome. Pathol. Res. Pract. 192, 271– 280 (1996).
Metoki, K. & Hommes, F.A. The pH of mitochondria of fibroblasts from a hyperornithinaemia, hyperammonaemia, homocitrullinuria syndrome patient. J. Inherit. Metab. Dis. 7, 9– 11 (1984).
Yokota, S. & Mori, M. Immunoelectron microscopical localization of ornithine transcarbamylase in hepatic parenchymal cells of the rat. Histochem. J. 18, 451–457 (1986).
Powers-Lee, S.G., Mastico, R.A. & Bendayan, M. The interaction of rat liver carbamoyl phosphate synthetase and ornithine transcarbamoylase with inner mitochondrial membranes. J. Biol. Chem. 262, 15683–15688 (1987).
Cheung, C.-W., Cohen, N.S. & Raijman, L. Channeling of urea cycle intermediates in situ in permeabilized hepatocytes. J. Biol. Chem. 264, 4038–4044 (1989).
Hendrick, J.P., Hodges, P.E. & Rosenberg, L.E. Survey of amino-terminal proteolytic cleavage sites in mitochondrial precursor proteins: Leader peptides cleaved by two matrix proteases share a three-amino acid motif. Proc. Natl Acad. Sci. USA 86, 4056–4060 ( 1989).
Brody, L.C. et al. Ornithine-δ-aminotransferase mutations causing gyrate atrophy: allelic heterogeneity and functional consequences. J. Biol. Chem. 267, 330–3307 (1992).
Acknowledgements
We thank B. Soares for supplying EST AH007255, J. Proffitt and Vysis, Inc. for providing the 13qtel probe and S. Muscelli for assistance in preparing the manuscript. J.A.C. is supported by an NIGMS Postdoctoral Training Grant (GM07471). Part of this work was supported by a grant from the National Eye Institute (EY07414) to D.V. and NICHD Mental Retardation Research Center Core Grant (HD24061) to B.K.G.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Camacho, J., Obie, C., Biery, B. et al. Hyperornithinaemia- hyperammonaemia- homocitrullinuria syndrome is caused by mutations in a gene encoding a mitochondrial ornithine transporter. Nat Genet 22, 151–158 (1999). https://doi.org/10.1038/9658
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/9658
This article is cited by
-
Comprehensive bioinformatics analysis of the solute carrier family and preliminary exploration of SLC25A29 in lung adenocarcinoma
Cancer Cell International (2023)
-
Metabolomics analysis of plasma samples of patients with fibromyalgia and electromagnetic sensitivity using GC–MS technique
Scientific Reports (2022)
-
SLC gene mutations and pediatric neurological disorders: diverse clinical phenotypes in a Saudi Arabian population
Human Genetics (2022)
-
Human cytosolic transaminases: side activities and patterns of discrimination towards physiologically available alternative substrates
Cellular and Molecular Life Sciences (2022)
-
CUGC for hyperornithinemia-hyperammonemia-homocitrullinuria (HHH) syndrome
European Journal of Human Genetics (2020)