Abstract
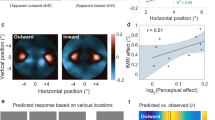
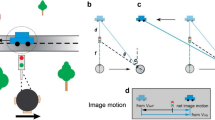
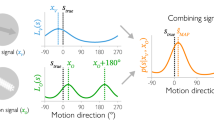
To perceive the relative positions of objects in the visual field, the visual system must assign locations to each stimulus. This assignment is determined by the object's retinal position, the direction of gaze, eye movements, and the motion of the object itself. Here we show that perceived location is also influenced by motion signals that originate in distant regions of the visual field. When a pair of stationary lines are flashed, straddling but not overlapping a rotating radial grating, the lines appear displaced in a direction consistent with that of the grating's motion, even when the lines are a substantial distance from the grating. The results indicate that motion's influence on position is not restricted to the moving object itself, and that even the positions of stationary objects are coded by mechanisms that receive input from motion-sensitive neurons.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Matin, L. in Handbook of Sensory Physiology VII/4 (eds. Jameson, D. & Hurvich, L.) 331–380 (Springer, Berlin, 1972).
Ross, J., Morrone, C. & Burr, D. C. Compression of visual space before saccades. Nature 386, 598–601 (1997).
Cai, R. H., Pouget, A., Schlag-Rey, M. & Schlag, J. Perceived geometrical relationships affected by eye-movement signals. Nature 386, 601–604 (1997).
Deubel, H., Schneider, W. X. & Bridgeman, B. Postsaccadic target blanking prevents saccadic suppression of image displacement. Vision Res. 36, 985 –996 (1996).
Frohlich, F. W. Die Empfindungszeit (Verlag von Gustav Fischer, Jena, 1929).
Matin, L., Boff, K. & Pola, J. Vernier offset produced by rotary target motion. Percept. Psychophys. 20, 138–142 (1976).
Nijhawan, R. Motion extrapolation in catching. Nature 370, 256–257 (1994).
Baldo, M. V. & Klein, S. A. Extrapolation or attention shift? Nature 378, 565–566 (1995).
Khurana, B. & Nijhawan, R. Extrapolation or attention shift? reply. Nature 378, 566 (1995).
Ramachandran, V. S. & Anstis, S. M. Illusory displacement of equiluminous kinetic edges. Perception 19, 611–616 (1990).
DeValois, R. L. & DeValois, K. K. Vernier acuity with stationary moving gabors. Vision Res. 31, 1619–1626 (1991).
Snowden, R. J. Shifts in perceived position following adaptation to visual motion. Curr. Biol. 8, 1343–1345 (1998).
Nishida, S. & Johnston, A. Influence of motion signals on the perceived position of spatial pattern. Nature 397 , 610–612 (1999).
Schlag, J., Cai, R., Dorfman, A., Mohempour, A. & Schlag-Rey, M. Extrapolating movement without retinal motion. Nature 403, 38–39 (2000).
Freyd, J. J. & Finke, R. A. Representational momentum. J. Exp. Psychol. 19, 369–401 (1987).
Purushothaman, G., Patel, S. S., Bedell, H. E. & Ogmen, H. Moving ahead through differential visual latency. Nature 396, 424 (1998).
Whitney, D. & Murakami, I. Latency difference, not spatial extrapolation. Nat. Neurosci. 1, 656– 657 (1998).
Whitney, D., Murakami, I. & Cavanagh, P. Illusory spatial offset of a flash relative to a moving stimulus is caused by differential latencies for moving and flashed stimuli . Vision Res. 40, 137–149 (2000).
Eagleman, D. M. & Sejnowski, T. J. Motion integration and postdiction in visual awareness. Science 287, 2036–2038 (2000).
Berry, M. J. II, Brivanlou, I. H., Jordan, T. A. & Meister, M. Anticipation of moving stimuli by the retina. Nature 398, 334–338 (1999).
Levi, D. M. & Klein, S. A. The role of separation and eccentricity in encoding position. Vision Res. 30, 557 –585 (1990).
Dichgans, J., Held, R., Young, L. R. & Brandt, T. Moving visual scenes influence the apparent direction of gravity. Science 178, 1217–1219 (1972).
Kertesz, A. & Jones, R. The effect of angular velocity of stimulus on human torsional eye movements. Vision Res. 9, 995–998 (1969).
Merker, B. H. & Held, R. Eye torsion and the apparent horizon under head tilt and visual field rotation. Vision Res. 21, 543–547 (1981).
Hughes, P. C., Brecher, G. & Fishkin, S. Effects of rotating backgrounds upon the perception of verticality. Percept. Psychophys. 11, 135–138 (1972).
Witkin, H. & Asch, S. Studies in space orientation. IV. Further experiments on perception of the up-right with displaced visual fields. J. Exp. Psychol. 38, 762–782 (1948).
Rock, I. in The Legacy of Solomon Asch (ed. Rock, I.) 243– 268 (Lawrence Erlbaum, Hillsdale, New Jersey, 1990).
Ramachandran, V. S. Interaction between colour and motion in human vision. Nature 328, 645–647 (1987).
Cropper, S. J. & Derrington, A. M. Motion of chromatic stimuli: first-order or second-order? Vision Res. 34, 49–58 (1994).
Cavanagh, P. Attention-based motion perception. Science 257, 1563–1565 (1992).
Kelly, D. H. Motion and vision. II. Stabilized spatio-temporal threshold surface. J. Opt. Soc. Am. 69, 1340–1349 (1979).
Nakayama, K. Biological image motion processing: a review. Vision Res. 25, 625–660 (1985).
Verstraten, F., Cavanagh, P. & Labianca, A. Limits of attentive tracking reveal temporal properties of atention. Vision Res. (in press).
Duncker, K. in Source Book of Gestalt Psychology (ed. and trans. Ellis, W. D.) 161–172 (Routledge & Kegan Paul, London, 1938). [Reprinted from Psychologische Forschung 12, 180–259, 1929].
Anstis, S. M. & Reinhardt-Rutland, A. H. Interactions between motion aftereffects and induced movement. Vision Res. 16, 1391–1394 (1976).
Nakayama, K. & Silverman, G. Temporal and spatial characteristics of the upper displacement limit for motion in random dots. Vision Res. 24, 293–300 (1984).
Snowden, R. J. & Braddick, O. J. Extension of displacement limits in multiple-exposure sequences of apparent motion. Vision Res. 29, 1777–1787 (1989).
Nawrot, M. & Sekuler, R. Assimilation and contrast in motion perception: explorations in cooperativity. Vision Res. 30, 1439–1451 (1990).
Reinhardt-Rutland, A. H. Induced movement in the visual modality: An overview. Psychol. Bull. 103, 57–71 (1988).
Gogel, W. C. & Koslow, M. The adjacency principle and induced movement. Percept. Psychophys. 11, 309– 314 (1972).
Felleman, D. J. & Kaas, J. H. Receptive-field properties of neurons in middle temporal area (MT) of owl monkeys. J. Neurophysiol. 52, 488–513 (1984).
Mikami, A., Newsome, W. T. & Wurtz, R. H. Motion selectivity in macaque visual cortex. II. Spatiotemporal range of directional interactions in MT and VI. J. Neurophysiol. 55 1328–1339 (1986).
Tanaka, K. & Saito, H. Analysis of motion of the visual field by direction, expansion/contraction, and rotation cells clustered in the dorsal part of the medial superior temporal area of the macaque monkey. J. Neurophysiol. 62, 626–641 (1989).
Maunsell, J. H. & Van Essen, D. C. Functional properties of neurons in middle temporal visual area of the macaque monkey. II. Binocular interactions and sensitivity to binocular disparity. J. Neurophysiol. 49, 1148–1167 (1983).
Maunsell, J. H. & Van Essen, D. C. Functional properties of neurons in middle temporal visual area of the macaque monkey. I. Selectivity for stimulus direction, speed, and orientation. J. Neurophysiol. 49, 1127–1147 (1983).
Tootell, R. B. et al. Visual motion aftereffect in human cortical area MT revealed by functional magnetic resonance imaging. Nature 375 , 139–141 (1995).
Zeki, S. in Coding and Efficiency (ed Blakemore, C.) 321– 345 (Cambridge Univ Press, Cambridge, 1990).
Daniel, P. M. & Whitteridge, D. The representation of the visual field on the cerebral cortex in monkeys. J. Physiol. (Lond.) 159, 203–221 (1961).
Edelman, G. M. in The Mindful Brain (eds. Edelman, G. M. & Mountcastle, V. B.) 55–100 (MIT Press, Cambridge, Massachusetts, 1978).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Whitney, D., Cavanagh, P. Motion distorts visual space: shifting the perceived position of remote stationary objects. Nat Neurosci 3, 954–959 (2000). https://doi.org/10.1038/78878
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/78878
This article is cited by
-
Gravitational effects of scene information in object localization
Scientific Reports (2021)
-
Distinct temporal developments of visual motion and position representations for multi-stream visuomotor coordination
Scientific Reports (2019)
-
Rapid and specific processing of person-related information in human anterior temporal lobe
Communications Biology (2019)
-
Apparent shift in long-range motion trajectory by local pattern orientation
Scientific Reports (2018)
-
Motion-induced position shift in early Alzheimer’s disease
Scientific Reports (2018)